This policy applies to all Pharmacy or non-pharmacy personnel accessing controlled work areas located within Lower Mainland Pharmacy Services.
|
|
|
- Stephen Welch
- 6 years ago
- Views:
Transcription
1 Page 1 of 13 BACKGROUND Proper hand hygiene and garbing are an essential part of the sterile compounding process to prevent patient harm from contaminated compounded sterile preparations and protect staff from drug and chemical exposure. PURPOSE To establish standard processes and outline requirements for hand hygiene and garbing for personnel working in or entering controlled work areas, in accordance with provincial legislation and standards. FOCUS This policy applies to all Pharmacy or non-pharmacy personnel accessing controlled work areas located within Lower Mainland. DEFINITIONS 2,3,4 Anteroom: An area ISO Class 7 or 8 area where personnel hand hygiene and garbing procedures, staging of components, order entry, compounded sterile product labeling, and other high-particulate-generating activities are performed. It is also a transition area that (1) provides assurance that pressure relationships are constantly maintained so that air flows from clean to dirty areas and (2) reduces the need for the heating, ventilating, and air-conditioning (HVAC) control system to respond to large disturbances. Cleanroom: An enclosed, ISO Class 7 area where the primary clean air device (e.g. cabinet, isolator, workbench, hood) is physically located. Compounded sterile preparation (CSP): A preparation intended to be sterile that is created by combining, diluting, pooling, or otherwise altering a drug product or bulk drug substance. Controlled work areas: includes anteroom, cleanroom, and segregated compounding area ISO Class: An air quality classification from the International Organization for Standardization.
2 Page 2 of 13 Line of demarcation: A line, real or virtual, that separates the anteroom or segregated compounding area into two spaces. The dirty side of the line of demarcation is located at the entrance to the anteroom, in the section adjacent to the pharmacy. The clean side of the line is adjacent to the dirty area on one side and the cleanroom on the other. It is important to take these clean and dirty areas into account when traversing the anteroom and when donning and removing personal protective equipment. Personal Protective Equipment (PPE): Equipment or clothing worn to minimize exposure to chemical hazards in the workplace, enable compliance with the expected specifications of a controlled environment and minimize potential contamination of CSPs. Primary clean air device: A device (e.g. cabinet, isolator, workbench, hood) that provides an ISO Class 5 environment for exposure of critical sites during the compounding of aseptic preparations. Segregated compounding area: A designated, unclassified space, area, or room that contains a primary clean air device for preparation of CSPs and is void of activities and materials that are extraneous to sterile compounding. POLICY General 1.1 Only authorized personnel shall enter controlled work areas 1.2 Personnel requiring access to controlled work areas shall report to the supervisor any illnesses or conditions that may adversely affect the safety or integrity of compounded sterile preparations (CSPs) as a result of excessive skin shedding or infectiousness Such illnesses or conditions include but are not limited to the following: Fever Moderate to severe sunburn with skin sloughing Visible or exposed eczema or other severe skin rash Cough, runny nose or active respiratory infection Conjunctivitis Open wounds or weeping sores, including recent tattoos Other active communicable diseases
3 Page 3 of The supervisor shall exclude affected personnel from accessing the controlled work area until the illness or condition is remedied or the potential impact on CSP safety and integrity has abated 1.3 Personnel accessing controlled work areas shall pay particular attention to personal hygiene which can affect CSP quality and the health and safety of patients Nails shall be clean and trimmed 1.4 While working in or accessing controlled work areas, personnel shall comply with hand hygiene and garb requirements as outlined in the procedures Applicable hand hygiene and garbing shall be performed each time personnel enter and leave the anteroom, cleanroom or segregated compounding area. 1.5 No food or drink, including chewing gum, candy or lozenges, shall be brought into, stored or consumed in controlled work areas Orientation 1.6 All personnel who enter controlled work areas shall receive orientation and successfully complete a hand hygiene and garbing competency assessment, prior to entering the controlled work area (See Appendix A) The hand hygiene and garbing competency assessment shall be completed annually 1.7 Personnel who have not received orientation or have not completed the competency assessment may access controlled work areas, only under the following circumstances: Must be accompanied by an oriented and qualified staff member, and Must be assisted through the complete hand hygiene and garbing process by an oriented and qualified staff member Personal Apparel 1.8 While working in or accessing controlled work areas, personnel shall not be permitted to wear the following: personal outer garments (e.g., bandanas, coats, hats, jackets, scarves, sweaters, vests)
4 Page 4 of 13 all leave-in hair products (e.g. hairspray, mousse, gel) and facial cosmetics as they shed flakes and particles (exception: non-tinted skin and lip moisturizers) all hand, wrist, neck and head jewelry nail polish and artificial nails or nail tips eyelash enhancements 1.9 While working in or accessing controlled work areas, personnel are permitted to wear the following: Eyeglasses Hearing aids Medical information, secured under the neckline of garb ID badges, secured under garb (lanyards are not permitted) Short sleeved undershirt, worn under garb 1.10 Personal electronic devices, including cell phones, music players and ear buds shall not be permitted inside controlled work areas, even when contained within a pocket Garb 1.11 While accessing controlled work areas, all personnel shall wear: Clean, low-shedding apparel Clean footwear in good condition, completely enclosing the foot from heel to toe, including covering the top side of the foot Socks or stockings covering the ankles, so that skin below the hem of the garb (scrubs) is completely covered 1.12 All personnel accessing controlled work areas beyond the line of demarcation (i.e. clean side of anteroom or segregated compounding area, cleanroom) shall wear fresh, dedicated, low-shedding apparel (i.e. scrubs) for each shift A fastened lab coat or gown shall be worn over the apparel upon exiting the controlled work area if personnel intend to reenter the controlled work area wearing the same apparel Gowns worn outside controlled work areas may not be reused upon reentering controlled work areas.
5 Page 5 of All personnel shall wear disposable or re-usable gowns in cleanrooms For hazardous drug compounding, only LMPS-approved disposable gowns resistant to fluid permeation shall be worn 1.14 Scrubs and re-usable gowns shall be Supplied by the hospital, Stored on site, and Laundered by the hospital laundry service 1.15 Eye-shields, in the form of goggles, are required for personnel performing an activity that has a high likelihood of splashing harmful substances such as cleaning products or hazardous materials Such activities include preparation of cleaning solutions, cleaning of controlled work area ceilings and walls, cleaning or decontamination of the Biological Safety Cabinet (BSC) with the viewing window raised, or cleaning up a hazardous drug spill outside the BSC 1.16 A NIOSH-certified elastomeric or N95 respirator, fit-tested annually, shall be worn when cleaning or decontaminating the BSC with the viewing window raised, or when cleaning hazardous spills outside the BSC Gloves 1.17 All personnel preparing CSPs shall wear sterile gloves personnel preparing drugs in the hazardous drug Biological Safety Cabinet shall wear two sets of sterile LMPS-approved chemotherapy gloves, with the inner pair worn under the gown cuff and the outer pair worn over the gown cuff Sterile gloves shall be worn over the isolator gloves inside the main chamber of an isolator 1.18 Personnel entering the cleanroom to perform tasks other than compounding, including observation, final verification of CSPs, cleaning areas other than the interior of the primary clean air device, and maintenance of equipment, may wear single-use nonsterile gloves donned after aseptic hand washing
6 Page 6 of The non-sterile gloves must be disinfected with sterile 70% isopropyl alcohol (sipa) after donning Non-sterile gloves worn in the hazardous drug cleanroom must be chemotherapy-approved PROCEDURE Personnel must don garb and perform hand hygiene in an order that proceeds from the dirtiest to cleanest body parts, top to bottom (head and face, feet), and takes into account the location of the line of demarcation in the facility. Each site will establish a specific order and location for hand hygiene and garbing activities. Prior to Entering the Anteroom or Segregated Compounding Area 2.1 Remove outer garments, jewelry, cosmetics 2.2 Don clean, low-shedding clothing (e.g. scrubs) 2.3 Perform routine hand hygiene (i.e. non surgical) using soap and water or alcohol based hand rub 2.4 If worn, clean eyeglasses with a low-shedding single-use cloth and eyeglass cleaning solution, sterile 70% isopropyl alcohol (sipa), soap and water or other disinfectant compatible with eyeglass materials In Anteroom or Upon Entry to Segregated Compounding Area Don Garb 2.5 Don head cover and beard cover, if required, ensuring all hair and ears are covered 2.6 Don surgical or respirator mask, ensuring coverage from the bridge of the nose to the area under the chin 2.7 Don shoe covers one at a time, or don clean shoes dedicated to the clean side of the ante-area and cleanroom only while stepping over the line of demarcation
7 Page 7 of A second pair of shoe covers should be donned if entering the hazardous cleanroom to minimize hazardous drug contamination of controlled work areas. The second pair can be donned while crossing the line of demarcation or on the clean side of the anteroom. The second pair should be removed immediately before or after exiting the hazardous drug cleanroom. Exception: A single pair of shoe covers is sufficient if the location of the line of demarcation results in both pairs of shoe covers being removed in the same location Shoe covers are single use and are not to be worn beyond the line of demarcation If used, dedicated shoes must be cleaned and disinfected weekly Perform Hand Hygiene 2.8 Wash hands and forearms up to the elbow for 30 to 60 seconds with neutral soap and water, ensuring lather is produced Measure minimum of 30 seconds by referring to the second hand of a clock or other objective means Clean nails with a disposable nail pick once each shift, during the first time that hand hygiene is performed Note: Use of nail and hand brushes is not recommended as they have been shown to cause micro-abrasions of the skin 2.9 Rinse thoroughly under running water 2.10 Dry hands and forearms thoroughly by patting with a low-shedding, single-use cloth 2.11 Turn off the water taps with the cloth and discard cloth, unless using a hands-free sink Notify supervisor if suffering from allergies or sensitivities to standard hand hygiene agents Consultation with Workplace Health and Infection Control will help determine an acceptable alternative solution
8 Page 8 of 13 Gown 2.13 Don a disposable or reusable gown that closes at the neck and has elastic cuffs. Push sleeves of gown up to prepare for application of alcohol-based hand rub For hazardous drugs, don an approved disposable gown that resists permeability On Clean Side of Anteroom or Upon Entry to the Cleanroom Gloving 2.14 Open the outer wrapper of the sterile glove package onto a clean surface, exposing the inner glove package (may occur prior to hand hygiene) 2.15 Apply alcohol-based hand rub (ABHR) with persistent activity to fingertips and all surfaces of forearms, lower gown sleeves, then apply ABHR to hands and fingers as per manufacturer s recommendations Alternatively, apply ABHR with persistent activity to fingertips and all surfaces of forearms, then don gown and reapply ABHR to hands and fingers as per ABHR manufacturer s recommendations 2.16 Allow hands to dry completely 2.17 Don sterile gloves Don the first glove with an ungloved hand by grabbing an inner surface of the glove, and sliding the hand into it and pulling it on Don the second glove by slipping the sterile gloved hand into the cuff of the second and placing the ungloved hand into the free glove Inspect both gloves for damage and replace if any defects (tears, holes and rips) are noted Ensure gloves cover cuffs of gown Gloves should be the last item donned before compounding begins When using an isolator to compound, don the sterile gloves over the isolator gloves rather than donning the sterile gloves in the anteroom area Don two pairs of sterile chemotherapy gloves for hazardous drug compounding. The cuff of the first pair goes under the gown cuff, and the cuff of the second pair goes over the gown cuff.
9 Page 9 of Sanitize all surfaces of gloved hands with sterile 70% isopropyl alcohol (sipa) Apply sipa to gloved hands and rub hands together to completely coat surfaces of gloves Allow sipa to dry completely Change Gloves 2.19 Change gloves: Every hour when preparing non-hazardous drugs Every 30 minutes when preparing hazardous drugs (both pairs) If tearing or a puncture, or contamination with hazardous drug occurs Repeat Hand Hygiene 2.20 Repeat full hand hygiene between glove changes Exception: Hands that are not visibly soiled may be cleansed with alcohol-based hand rub with persistent activity as per manufacturer s recommendations when performing non-hazardous sterile compounding Always perform full hand hygiene procedures when changing gloves due to tearing or a puncture Disinfecting Gloves 2.21 Disinfect gloved hands with sipa (wipe or pour onto gloves) at the following times: Prior to entering the primary clean air device (e.g. when placing items into the hood) Prior to commencing each new batch Any time the gloved hands re-enter the primary clean air device after making contact with non-sterile surfaces Periodically, during prolonged periods of compounding within the primary clean air device Upon Exiting the Clean Room or Segregated Compounding Area 2.22 All PPE used for hazardous compounding must be disposed of into hazardous waste receptacles
10 Page 10 of Remove gloves and discard For hazardous drug compounding, remove outer gloves inside the primary clean air device. Alternatively, outer gloves may be cleaned with a saturated wipe inside the primary clean air device and removed before exiting the cleanroom. Gloves and any wipes used shall be discarded in the hazardous waste receptacle For hazardous drug compounding, remove and dispose of inner gloves after removal of other garb in the anteroom For hazardous drug compounding, remove the outer second set of shoe covers immediately before or after exiting the hazardous drug cleanroom 2.25 Remove gown, prior to stepping over the line of demarcation into the dirty section of the anteroom Gown may be saved for subsequent use throughout the shift provided it is not visibly soiled. If retained, hang on a hook in the cleanroom or on the clean side of the anteroom. Gowns may not be shared between staff members Chemotherapy gowns must be changed every 3 hours of compounding, at minimum Chemotherapy gowns worn in the hazardous compounding cleanroom must be removed immediately before or after exiting the cleanroom to prevent the spread of hazardous drug contamination from one area to another At the end of the session or shift, discard disposable gown into appropriate waste container or place reusable gown in laundry 2.26 Remove remaining shoe covers, upon stepping over the line of demarcation into the dirty section of the anteroom, and discard 2.27 Remove and discard face mask, facial hair cover, and head cover on the dirty side of the anteroom or beyond the line of demarcation 2.28 Wash hands with soap and water
11 Page 11 of 13 REFERENCES 1. BCCA Pharmacy Practice Standards for Hazardous Drugs. BC Cancer Agency, Available from 2. Compounding: Guidelines for Pharmacies. Canadian Society of Hospital Pharmacists, Ottawa, Ontario, Model Standards for Pharmacy Compounding of Non-hazardous Sterile Products. National Association of Pharmacy Regulatory Authorities, United States Pharmacopeia, General Chapter <797>: Pharmaceutical compounding sterile preparations. USP 39, Rockville, MD, United States Pharmacopeia, General Chapter <800>: Hazardous Drugs handling in healthcare settings. USP 39, Rockville, MD,
12 Appendix A Page 12 of 13 Hand Hygiene and Garbing Competency Assessment Employee Name: Evaluator Name: Date of Evaluation: Position Title: Type: Initial Competency Ongoing Competency Other: Evaluator Position: Observations and evaluations must be made by qualified pharmacy staff. In the RATING column, the evaluator will note the following: S=satisfactory competency, U= unmet competency, N/A = not applicable, N/O= not observed Additional comment is required if any notations of U, N/A or N/O, with a specific plan to correct noted in the Remedial Plan section. SKILL RATING COMMENTS Presents in a clean appropriate attire. Removes extraneous personal clothing (scarves, vests, sweaters, hats, etc) and wears clothing that is consistent with established policy Wears no cosmetics or jewelry (watches, rings, earrings, piercings visible prior to garbing) upon entry into ante-area. Neither brings nor stores food, drink or personal electronic device in the controlled work areas. Nails are not excessively long, no artificial nails Wears appropriate shoes and socks covering ankles Changes into clean, low-shedding apparel i.e. scrubs Cleans eyeglasses, if worn Performs routine hand hygiene (soap + water or regular alcohol-based hand rub) prior to entering controlled work areas Demonstrates awareness of and performs garbing activities on correct side of the line of demarcation. Dons head cover, assures all hair covered using mirror to verify Dons face mask to cover bridge of nose down to base of chin Optional: Dons beard cover if necessary. Optional: Puts on safety goggles, if required. Dons shoe covers or designated clean shoes one at a time, placing the covered or designated shoe on the clean side of the line of demarcation as appropriate. Dons 2 pairs of shoe covers for hazardous drug compounding. Wets hands and forearms, washes using soap and water for at least 30 seconds during which nails are cleaned with disposable nail pick. Dries hands and forearms by patting with low-linting towel. Dons gown and ensures full closure. Gown may be reusable or disposable for non-hazardous compounding, but must be impervious and disposable for hazardous compounding.
13 Appendix A Page 13 of 13 SKILL RATING COMMENTS Disinfects hands again using a waterless alcohol-based surgical hand rub with persistent activity and allows hands to dry thoroughly before donning sterile gloves. Dons appropriate-sized sterile gloves. Dons 2 sets of gloves for hazardous compounding (under and over gown cuffs). Examines gloves ensuring that there are no defects or holes Routinely disinfects gloves with sterile 70% IPA prior to entering PCAD, routinely during compounding and after touching items or surfaces outside the PCAD Change gloves every hour during non-hazardous and every 30 minutes during hazardous drug compounding Performs hand hygiene between glove changes (ABHR or full hand hygiene for non-hazardous compounding or full hand hygiene for hazardous compounding) Removes gloves at end of compounding session. For hazardous drug compounding, removes outer gloves inside PCAD or wipes gloves in PCAD and removes outer pair in cleanroom. Removes inner pair after removing other hazardous compounding PPE Removes non-hazardous gown and discards it (if finished compounding for the day) or hangs on a hook in cleanroom or on the clean side of the ante-area (if not visibly soiled and is intact) where it may be reused during the same work day only Changes hazardous gown every 3 hours. Removes gown immediately before/after exiting hazardous drug cleanroom For hazardous compounding, removes outer pair of shoe covers immediately before/after exiting hazardous cleanroom. Removes remaining shoe covers or shoes one at a time when crossing line of demarcation, ensuring that the uncovered foot is placed on the dirty side of the line of demarcation Removes and discards mask, head cover and beard cover crossing over line of demarcation Disposes of all PPE for hazardous compounding in hazardous waste receptacles Washes hands with soap and water after removing garb Remedial Action Plan/Additional Comments Employee Signature Date Qualified Evaluator Signature Date
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Competency. Method of Instruction Codes. Yes / No / RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN.
 JOHNS HOPKINS MEDICAL INSTITUTIONS Competency Validation Tool BIOLOGICAL PPE ENHANCED CONTACT AND DROPLET PRECAUTIONS (POWERED AIR PURIFYING RESPIRATOR (PAPR) OPTION) Employee Name: Role: Unit: Date: DESIRED
JOHNS HOPKINS MEDICAL INSTITUTIONS Competency Validation Tool BIOLOGICAL PPE ENHANCED CONTACT AND DROPLET PRECAUTIONS (POWERED AIR PURIFYING RESPIRATOR (PAPR) OPTION) Employee Name: Role: Unit: Date: DESIRED
ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ]
![ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ] ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ]](/thumbs/78/77108755.jpg) Page 1 of 5 ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST Trained Observer: Unit: Date: Healthcare Worker: Assistant: Instructions: Check box after completion of each step. Donning of HEALTHCARE
Page 1 of 5 ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST Trained Observer: Unit: Date: Healthcare Worker: Assistant: Instructions: Check box after completion of each step. Donning of HEALTHCARE
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
COMPOUNDING FREQUENCY OF DOCUMENTATION AND CLEANING CHA/CSHP Interpretation of California Board of Pharmacy (1/1/17) and USP<797> (2008) Requirements
 Page 1 of 1 TEMPERATURE REQUIREMENTS AND MONITORING CHA/CSHP Interpretation of California Board of Pharmacy (1/1/17) and USP(2008) Requirements) Temperature Description Controlled Freezer Temperature
Page 1 of 1 TEMPERATURE REQUIREMENTS AND MONITORING CHA/CSHP Interpretation of California Board of Pharmacy (1/1/17) and USP(2008) Requirements) Temperature Description Controlled Freezer Temperature
10/2/15. Charles Logan, CPhT
 10/2/15 Discuss USP 797 importance of workflow in the sterile compounding area. Recognize the proper procedures to be followed when compounding sterile preparations. PPE Charles Logan, CPhT Cleaning the
10/2/15 Discuss USP 797 importance of workflow in the sterile compounding area. Recognize the proper procedures to be followed when compounding sterile preparations. PPE Charles Logan, CPhT Cleaning the
Hand Hygiene. Policy Title: Hand Hygiene Policy Number: 05. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
CHAPTER 13 PROCEDURE checklists
 PROCEDURE checklists 179 CHAPTER 13 PROCEDURE checklists Procedure 13-1 Handwashing 1. Gather needed supplies if not present at the handwashing area: soap or the cleansing agent specified by your facility,
PROCEDURE checklists 179 CHAPTER 13 PROCEDURE checklists Procedure 13-1 Handwashing 1. Gather needed supplies if not present at the handwashing area: soap or the cleansing agent specified by your facility,
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Ventricular Assist Device (VAD) Exit Site Care Guidelines
 Ventricular Assist Device (VAD) Exit Site Care Guidelines I. STATEMENT OF PURPOSE The Ventricular Assist Device (VAD) Exit Site Care Guidelines are intended to provide standardization and continuity of
Ventricular Assist Device (VAD) Exit Site Care Guidelines I. STATEMENT OF PURPOSE The Ventricular Assist Device (VAD) Exit Site Care Guidelines are intended to provide standardization and continuity of
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
HAND HYGIENE QUIZ. 1. Why is hand hygiene so important? (1 point) a. It is one of the single most effective measures for
 HAND HYGIENE QUIZ 1. Why is hand hygiene so important? (1 point) a. It is one of the single most effective measures for preventing the spread of infection. 2. Name five reasons why staff do not wash their
HAND HYGIENE QUIZ 1. Why is hand hygiene so important? (1 point) a. It is one of the single most effective measures for preventing the spread of infection. 2. Name five reasons why staff do not wash their
Current Status: Active PolicyStat ID: Original Policy: 10/1986 Last Reviewed: 01/2016 Last Revised: 01/2016 Next Review: 01/2019
 Current Status: Active PolicyStat ID: 2085666 Original Policy: 10/1986 Last Reviewed: 01/2016 Last Revised: 01/2016 Next Review: 01/2019 Owner: Policy Area: References: Applicability: Tracy Dodson: Director
Current Status: Active PolicyStat ID: 2085666 Original Policy: 10/1986 Last Reviewed: 01/2016 Last Revised: 01/2016 Next Review: 01/2019 Owner: Policy Area: References: Applicability: Tracy Dodson: Director
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Hand Hygiene ORGANIZATIONAL: Affects two or more departments.
 Hand Hygiene ORGANIZATIONAL: Affects two or more departments. Folder Infection Prevention Sub-Folder Original 1/1/1987 Scope All Effective Date Approved (Approver/Date) Last Reviewed/ Revised Date IPC:
Hand Hygiene ORGANIZATIONAL: Affects two or more departments. Folder Infection Prevention Sub-Folder Original 1/1/1987 Scope All Effective Date Approved (Approver/Date) Last Reviewed/ Revised Date IPC:
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
VWR Garments for Laboratories and Clean Rooms!
 for Laboratories and Clean Rooms! Protection from Head to Toe - exclusively from Bio-Strategy! A wide range of laboratory and clean room apparel. Clean room garments are manufactured at special ISO accredited
for Laboratories and Clean Rooms! Protection from Head to Toe - exclusively from Bio-Strategy! A wide range of laboratory and clean room apparel. Clean room garments are manufactured at special ISO accredited
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
VWR Garments for Laboratories and Clean Rooms!
 for Laboratories and Clean Rooms! Protection from Head to Toe - exclusively from Bio-Strategy! A wide range of laboratory and clean room apparel. Clean room garments are manufactured at special ISO accredited
for Laboratories and Clean Rooms! Protection from Head to Toe - exclusively from Bio-Strategy! A wide range of laboratory and clean room apparel. Clean room garments are manufactured at special ISO accredited
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
2.2 Body protection consists of torso, hand, head, respiratory and foot protection.
 Vashon Island Fire & Rescue Policies and Operating Guidelines Policy: PERSONAL PROTECTIVE CLOTHING Number Effective Date Approved and Issued: 1701 01/04/00 01/04/00 1.0 REFERENCE WAC 296-305-02001-02009
Vashon Island Fire & Rescue Policies and Operating Guidelines Policy: PERSONAL PROTECTIVE CLOTHING Number Effective Date Approved and Issued: 1701 01/04/00 01/04/00 1.0 REFERENCE WAC 296-305-02001-02009
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Originated By: Human Resources Original Date: June 1, 1991
 POLICY No: (p) Originated By: Human Resources Original Date: June 1, 1991 Policy Owner(s): Human Resources Last Review Date: July, 2013 Last Revised Date: 10/2012 4/1997 10/1999 11/1992 9/2001 2/2005 6/2001
POLICY No: (p) Originated By: Human Resources Original Date: June 1, 1991 Policy Owner(s): Human Resources Last Review Date: July, 2013 Last Revised Date: 10/2012 4/1997 10/1999 11/1992 9/2001 2/2005 6/2001
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS
 PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER SANITARY REQUIREMENTS TABLE OF CONTENTS
 RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER 0200-03 SANITARY REQUIREMENTS TABLE OF CONTENTS 0200-03-.01 Applicability 0200-03-.02 Violations 0200-03-.03 Location 0200-03-.04 Communicable
RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER 0200-03 SANITARY REQUIREMENTS TABLE OF CONTENTS 0200-03-.01 Applicability 0200-03-.02 Violations 0200-03-.03 Location 0200-03-.04 Communicable
What is infection control?
 Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
Protective Clothing and Medical Devices
 Technical Guide Protective Clothing and Medical A technical guide for clothing manufacturers of garments for medical use. Published Standards EN 13795:2011+A1:2013 Surgical drapes, gowns and clean air
Technical Guide Protective Clothing and Medical A technical guide for clothing manufacturers of garments for medical use. Published Standards EN 13795:2011+A1:2013 Surgical drapes, gowns and clean air
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Policy: C-08-PRO Reviewed: 7/08
 Written: 1979 Policy: Reviewed: 7/08 Procedure Policy Manual Revised: 81, 84, 85, 86, 87, 88, 89, 90, 92, 93, 95 Ambulatory Care Division 2/97, 7/98, 7/00, 7/02, 7/04, 7/06, 2/09, 7/10 LSU Health Sciences
Written: 1979 Policy: Reviewed: 7/08 Procedure Policy Manual Revised: 81, 84, 85, 86, 87, 88, 89, 90, 92, 93, 95 Ambulatory Care Division 2/97, 7/98, 7/00, 7/02, 7/04, 7/06, 2/09, 7/10 LSU Health Sciences
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CULINARD. Dress To Success
 CULINARD Dress To Success CULINARD UNIFORM POLICY As a student at Culinard you are expected to demonstrate professionalism and good judgment at all times related to your appearance. Students must wear
CULINARD Dress To Success CULINARD UNIFORM POLICY As a student at Culinard you are expected to demonstrate professionalism and good judgment at all times related to your appearance. Students must wear
HAND HYGIENE PROCEDURE ICPR010
 HAND HYGIENE PROCEDURE ICPR010 Version No. Date Ratified/ Amended Date of Implementation Next Review Date Reason for Change (eg. full rewrite, amendment to reflect new legislation, updated flowchart, minor
HAND HYGIENE PROCEDURE ICPR010 Version No. Date Ratified/ Amended Date of Implementation Next Review Date Reason for Change (eg. full rewrite, amendment to reflect new legislation, updated flowchart, minor
Table 6: Detailed Infection Prevention and Control Procedures for Tattooing and Micropigmentation. Use During Tattooing
 FACT SHEET Table 6: Detailed Infection Prevention and Control Procedures for and Micropigmentation 1. Skin Preparation Spray bottle with a solution of soap and water Single use disposable razor The skin
FACT SHEET Table 6: Detailed Infection Prevention and Control Procedures for and Micropigmentation 1. Skin Preparation Spray bottle with a solution of soap and water Single use disposable razor The skin
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
CONSOLIDATION UPDATE: DECEMBER 11, 2002
 REPEALED BY THE BODY MODIFICATION BY-LAW NO. 40/2005 MARCH 23, 2005 (effective January 1, 2006) CONSOLIDATION UPDATE: DECEMBER 11, 2002 THE CITY OF WINNIPEG TATTOO STUDIO BY-LAW NO. 4653/87 A By-law of
REPEALED BY THE BODY MODIFICATION BY-LAW NO. 40/2005 MARCH 23, 2005 (effective January 1, 2006) CONSOLIDATION UPDATE: DECEMBER 11, 2002 THE CITY OF WINNIPEG TATTOO STUDIO BY-LAW NO. 4653/87 A By-law of
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
APPROVAL REVIEW PROCEDURES
 Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
AFS Environmental Health & Safety Conference Nashville, TN August 24, 2010
 AFS Environmental Health & Safety Conference Nashville, TN August 24, 2010 Protect employees from illness and injury associated with the use of hazardous substances A generic and performance oriented standard
AFS Environmental Health & Safety Conference Nashville, TN August 24, 2010 Protect employees from illness and injury associated with the use of hazardous substances A generic and performance oriented standard
Regulations Governing Barber and Beauty Culture Establishments, 1979
 BARBER AND BEAUTY CULTURE 1 Regulations Governing Barber and Beauty Culture Establishments, 1979 Repealed by Chapter P-37.1 Reg 10 (effective December 5, 2002). Formerly Saskatchewan Regulations 213/79
BARBER AND BEAUTY CULTURE 1 Regulations Governing Barber and Beauty Culture Establishments, 1979 Repealed by Chapter P-37.1 Reg 10 (effective December 5, 2002). Formerly Saskatchewan Regulations 213/79
Chino Valley Independent Fire District Tim Shackelford, Fire Chief
 Chino Valley Independent Fire District Tim Shackelford, Fire Chief Standard Operating Procedure: Administration SOP #108.01 Grooming Standard PURPOSE: Chino Valley Fire District is a professional organization,
Chino Valley Independent Fire District Tim Shackelford, Fire Chief Standard Operating Procedure: Administration SOP #108.01 Grooming Standard PURPOSE: Chino Valley Fire District is a professional organization,
LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard
 ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
Technical Information. Clorox Healthcare Bleach Germicidal Cleaner OVERVIEW. Clorox Healthcare Bleach Germicidal
 OVERVIEW Bleach Germicidal Cleaner is engineered as a ready-to-use sporicidal cleaner-disinfectant system for healthcare facilities. This EPA-registered disinfectant contains sodium hypochlorite and other
OVERVIEW Bleach Germicidal Cleaner is engineered as a ready-to-use sporicidal cleaner-disinfectant system for healthcare facilities. This EPA-registered disinfectant contains sodium hypochlorite and other
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
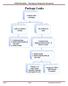 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Scabies Identification, Treatment and Environmental Cleaning
 Scabies Identification, Treatment and Environmental Cleaning Level III Purpose The purpose of this procedure is to treat residents infected with and sensitized to Sarcoptes scabiei and to prevent the spread
Scabies Identification, Treatment and Environmental Cleaning Level III Purpose The purpose of this procedure is to treat residents infected with and sensitized to Sarcoptes scabiei and to prevent the spread
Hazard Communication Program
 1. Purpose The University of Denver Hazard Communication Program defines the requirements and responsibilities for informing and training employees about workplace hazardous chemicals in accordance with
1. Purpose The University of Denver Hazard Communication Program defines the requirements and responsibilities for informing and training employees about workplace hazardous chemicals in accordance with
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
GENERAL ASSEMBLY OF NORTH CAROLINA SESSION 2001 H 1 HOUSE BILL 635. March 15, 2001
 GENERAL ASSEMBLY OF NORTH CAROLINA SESSION 00 H HOUSE BILL Short Title: Regulate Body Piercing. Sponsors: Representatives Mitchell; Capps and Setzer. Referred to: Finance. (Public) March, 00 0 A BILL TO
GENERAL ASSEMBLY OF NORTH CAROLINA SESSION 00 H HOUSE BILL Short Title: Regulate Body Piercing. Sponsors: Representatives Mitchell; Capps and Setzer. Referred to: Finance. (Public) March, 00 0 A BILL TO
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
BODY ART ESTABLISHMENT INTRODUCTION GUIDE
 BODY ART ESTABLISHMENT INTRODUCTION GUIDE Toledo-Lucas County Health Department 635 N. Erie Street Toledo, OH 43604 Phone: (419) 213-4100 ext. 3 Fax: (419) 213-4141 INTRODUCTION This guide has been developed
BODY ART ESTABLISHMENT INTRODUCTION GUIDE Toledo-Lucas County Health Department 635 N. Erie Street Toledo, OH 43604 Phone: (419) 213-4100 ext. 3 Fax: (419) 213-4141 INTRODUCTION This guide has been developed
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Hand Scrubs Gowning & Gloving
 2016 Aseptic Practices Chesapeake Bay Perioperative Consortium Hand Scrubs Gowning & Gloving Objectives The novice perioperative team member will be able to: Define difference between asepsis technique
2016 Aseptic Practices Chesapeake Bay Perioperative Consortium Hand Scrubs Gowning & Gloving Objectives The novice perioperative team member will be able to: Define difference between asepsis technique
BODY ART GUIDELINES. Purpose. Definitions. Body Art Technician Requirements
 BODY ART GUIDELINES Purpose This guideline provides general explanations of procedures for the maintenance and operation of body art facilities and permitting requirements for body art technicians. Please
BODY ART GUIDELINES Purpose This guideline provides general explanations of procedures for the maintenance and operation of body art facilities and permitting requirements for body art technicians. Please
Maintenance Guidelines Scuba
 Maintenance Code W/B-Clean with water-based cleanser or diluted household bleach. Regular Maintenance Vacuum regularly using the proper attachment to avoid pilling. For non-woven textiles, wipe regularly
Maintenance Code W/B-Clean with water-based cleanser or diluted household bleach. Regular Maintenance Vacuum regularly using the proper attachment to avoid pilling. For non-woven textiles, wipe regularly
Personal Care Caregiving Series: Volume 8
 Personal Care Caregiving Series: Volume 8 Objectives Upon completion of this training, the participant will understand: Procedures for providing personal hygiene The importance of the principles of body
Personal Care Caregiving Series: Volume 8 Objectives Upon completion of this training, the participant will understand: Procedures for providing personal hygiene The importance of the principles of body
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
EMS Standard Operating Procedures Policy Title:
 EMS Standard Operating Procedures Policy Title: Uniforms & Appearance Section: Operations Adoption Date: 07/24/2000 Date of Most Recent Update: 09/2017 CAAS Criteria Reference: Purpose: To provide guidelines
EMS Standard Operating Procedures Policy Title: Uniforms & Appearance Section: Operations Adoption Date: 07/24/2000 Date of Most Recent Update: 09/2017 CAAS Criteria Reference: Purpose: To provide guidelines
ALABAMA BOARD OF COSMETOLOGY ADMINISTRATIVE CODE CHAPTER 250-X-3 SALON REQUIREMENTS TABLE OF CONTENTS
 ALABAMA BOARD OF COSMETOLOGY ADMINISTRATIVE CODE CHAPTER 250-X-3 SALON REQUIREMENTS TABLE OF CONTENTS 250-X-3-.01 General Requirements 250-X-3-.02 Products Sanitation And Care 250-X-3-.03 Shops 250-X-3-.04
ALABAMA BOARD OF COSMETOLOGY ADMINISTRATIVE CODE CHAPTER 250-X-3 SALON REQUIREMENTS TABLE OF CONTENTS 250-X-3-.01 General Requirements 250-X-3-.02 Products Sanitation And Care 250-X-3-.03 Shops 250-X-3-.04
Procedure 19 Changing A Clean Dressing. Procedure 20 Applying A Bandage. Procedure 21 Applying A Sterile Dressing
 Chapter 5 Wound Care Procedure 19 Changing A Clean Dressing Procedure 20 Applying A Bandage Procedure 21 Applying A Sterile Dressing Procedure 22 Applying A Dressing Around A Drain Procedure 23 Changing
Chapter 5 Wound Care Procedure 19 Changing A Clean Dressing Procedure 20 Applying A Bandage Procedure 21 Applying A Sterile Dressing Procedure 22 Applying A Dressing Around A Drain Procedure 23 Changing
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Material Safety Data Sheet GFRC (Glass Fiber Reinforced Concrete)
 Material Safety Data Sheet GFRC (Glass Fiber Reinforced Concrete) SECTION 1 CHEMICAL PRODUCT AND IDENTIFICATION PRODUCT(S): GFRC; molded Glass Fiber Reinforced Concrete COMMON NAME I CHEMICAL FAMILY: Glass
Material Safety Data Sheet GFRC (Glass Fiber Reinforced Concrete) SECTION 1 CHEMICAL PRODUCT AND IDENTIFICATION PRODUCT(S): GFRC; molded Glass Fiber Reinforced Concrete COMMON NAME I CHEMICAL FAMILY: Glass
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. PRODUCT AND COMPANY IDENTIFICATION Product Chemical characterization Supplier Emergency telephone number Product use Product Identification Number Hepes Buffered Saline Solution
MATERIAL SAFETY DATA SHEET 1. PRODUCT AND COMPANY IDENTIFICATION Product Chemical characterization Supplier Emergency telephone number Product use Product Identification Number Hepes Buffered Saline Solution
Personal Care Caregiving Series
 Personal Care Caregiving Series Objectives Upon completion of this training, the participant will understand: Procedures for providing personal hygiene The importance of the principles of body mechanics
Personal Care Caregiving Series Objectives Upon completion of this training, the participant will understand: Procedures for providing personal hygiene The importance of the principles of body mechanics
Prosonic BS washable clean. trimmer. off. eco. normal. intensive. high. auto select. low. empty. reset
 on ActivePower Prosonic 9795 ing 9795 off reset BS 9795 www.braun.co.jp 2 3 STOP 4 5 6 7 8 9000 Series reset reset on off on off ing 3 4 6 5 1 2 4a 9 8 10 11 12 13 14 15 16 17 7 18 9795 9 10 11 on tr reset
on ActivePower Prosonic 9795 ing 9795 off reset BS 9795 www.braun.co.jp 2 3 STOP 4 5 6 7 8 9000 Series reset reset on off on off ing 3 4 6 5 1 2 4a 9 8 10 11 12 13 14 15 16 17 7 18 9795 9 10 11 on tr reset
RULES OF TENNESSEE STATE BOARD OF COSMETOLOGY CHAPTER SANITARY RULES TABLE OF CONTENTS
 RULES OF TENNESSEE STATE BOARD OF COSMETOLOGY CHAPTER 0440-2 SANITARY RULES TABLE OF CONTENTS 0440-2-.01 Definitions 0440-2-.10 Animals 0440-2-.02 Applicability 0440-2-.11 High Frequency Electric Current
RULES OF TENNESSEE STATE BOARD OF COSMETOLOGY CHAPTER 0440-2 SANITARY RULES TABLE OF CONTENTS 0440-2-.01 Definitions 0440-2-.10 Animals 0440-2-.02 Applicability 0440-2-.11 High Frequency Electric Current
Radiology Dress Guidelines. To establish minimal acceptable standards of dress for employees of Radiology
 Radiology Dress Guidelines Purpose: To establish minimal acceptable standards of dress for employees of Radiology Policy: 1. University Health identification badges must be worn while on duty, displayed
Radiology Dress Guidelines Purpose: To establish minimal acceptable standards of dress for employees of Radiology Policy: 1. University Health identification badges must be worn while on duty, displayed
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
Procedure/ Care Plan for Domiciliary Care Workers/ Support Workers - Application of Prescribed Creams/ Ointments/ Lotions (Adult)
 Application of Prescribed Creams/ Ointments/ Lotions (Adult) CLINICAL GUIDELINES ID TAG Medicines Management Specific Title: Procedure: Application of prescribed Creams/ Ointments/ Lotions (Adult) Author:
Application of Prescribed Creams/ Ointments/ Lotions (Adult) CLINICAL GUIDELINES ID TAG Medicines Management Specific Title: Procedure: Application of prescribed Creams/ Ointments/ Lotions (Adult) Author:
