Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
|
|
|
- Rudolf Townsend
- 6 years ago
- Views:
Transcription
1 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing documents established by the facility unless written permission has been obtained from the responsible facility manager. PREFACE The overall goal of infection prevention practices is to eliminate the risk of the transmission of pathogens between patients and between patients and the health care worker. The following recommendations should be implemented when cleaning and disinfecting. These procedures follow the Spaulding Classification of the level of care required for surfaces and instruments. Non-critical equipment is devices or equipment that comes in contact with intact skin but not mucous membranes. Intact skin acts as an effective barrier to most microorganisms. Examples of non-critical equipment are bedpans, blood pressure cuffs, and patient care equipment like keyboards and monitors. There is virtually no risk of transmitting infectious agents to patients via non-critical items; however, these items could potentially contribute to secondary transmission by contaminated hands of Health Care Workers or by contact with medical equipment that will subsequently come in contact with patients. Contaminated instruments classified as semi-critical or critical should be stored in a separate container/bag until back to the station or hospital. Contaminated instruments should be cleaned in the dirty zone, preferably in a utility sink. Cleaning removes soil and body materials, e.g. blood, from instruments, equipment and environmental surfaces. Cleaning must occur as an integral first step before disinfection or sterilization. Once cleaned, the instruments should be High Level Disinfected, Chemically Sterilized or Mechanically Sterilized according to Spaulding classification. PROTECTIVE BARRIERS 1. Disposable gloves. Gloves should be changed as required, i.e., when torn, when hands become wet inside the glove or when moving between patient rooms. 2. Household gloves can be worn, but they must be discarded when the cleaning is complete. 3. Protective Eye wear (goggles, face shield or mask with eye protection) 4. Masks (surgical or procedural masks sufficient) 5. Gowns PRODUCTS High Level Disinfection: PREVENTION a 2% Accelerated Hydrogen Peroxide Chemical Sterilization: Accel CS 20 a 7% Accelerated Hydrogen Peroxide Instrument Cleaning: Accel Wash a 3% Stabilized Hydrogen Peroxide Environmental Surfaces: Accelerated Hydrogen Peroxide Surface Disinfectant (sold as 7% Virox 5 Concentrate, Virox 5 Ready-To-Use and/ or Virox 5 Wipes, 7% PerCept Concentrate, PerCept RTU or PerCept Wipes, 7% Accel Surface Cleaner Disinfectant Concentrate, Accel RTU or Accel Wipes) and 0.5% Accelerated Hydrogen Peroxide Tuberculocidal Surface Disinfectant (sold as Accel TB RTU or Accel TB Wipes) 1. PREVENTION and Accel CS 20 are ready-to-use solutions. 2. Preparation of Accel Wash - Pre-mix and label from a controlled location 3% SHP Concentrate at a ratio of 1:128 into a wash basin or sonicating bath. 3. Preparation of solution - Pre-mix and label from a controlled location 7% AHP Concentrate at a ratio of 1:16 (0.5% AHP).
2 4. Place mixed solution in either a labeled - flip top 1 Litre bottle or a small hand bucket. 5. AHP RTU is ready to use (0.5% AHP). 6. AHP Wipes are ready to use (0.5% AHP). PRODUCT GERMICIDAL EFFICACY Accel CS20 is based upon patented Accelerated Hydrogen Peroxide (AHP). Accel CS20 is a 7% AHP High Level Disinfectant and Chemosterilant solution that carries a 20-minute Fungicidal, Tuberculocidal claim and Sporicidal claim. Accel CS20 has a 16-month shelf life with a 14-day reuse claim. PREVention is based upon patented Accelerated Hydrogen Peroxide (AHP). PREVention is a 2% AHP High Level Disinfectant solution that carries a 5-minute Bactericidal, Virucidal, Fungicidal and Tuberculocidal claim. Additionally, PREVention is also an approved Chemosterilant with proven Sporicidal efficacy in 6-hours. PREVention has a 12- month shelf life with a 14-day reuse claim. All Environmental Surface Products listed above are based upon Accelerated Hydrogen Peroxide and have a Broad- Spectrum Sanitizing claim against vegetative bacteria, a Bactericidal claim against gram negative and gram positive vegetative bacteria as well as General Virucide Claim against Poliovirus Type 1, Sabin Strain, which includes inactivation of both enveloped and non-enveloped viruses. In addition to the General Virucide Claim, Accelerated Hydrogen Peroxide has been proven to show efficacy against HIV, Human Coronavirus, Human Rhinovirus, Human Rotavirus, Canine Parvovirus, Feline Calicivirus (Norovirus) and the H3N2 strain of Avian Influenza A. The Tuberculocidal Surface Disinfectant (Oxivir Tb, Carpe Diem Tb and Accel TB) also carries a Fungicidal and Tuberculocidal claim. SUMMARY OF PROCEDURES FOR ENVIRONMENTAL SURFACES AND NON-CRITICAL DEVICES Apply AHP Solution to either surface or to cloth. Clean all horizontal surfaces in the room ensuring that the cloth is changed when soiled. Place used cloth in a marked plastic-lined waste receptacle. Disinfect all horizontal surface of the room by reapplying the AHP Solution and allowing for a 5-minute contact time. If using cloth & bucket method with double dipping, once room has been cleaned discard all unused cleaning solution before proceeding to the next room. Allow surfaces to air dry or wipe dry if surfaces are still wet after the 5-minute contact time. Periodic rinsing of soft surfaces such as vinyl or Naugahyde is suggested as well as equipment regularly handled by hand. Recommended Procedures for Cleaning and Disinfection of Environmental Surfaces and Patient Care Equipment Contaminated patient care devices should be clearly identified and kept separate from clean patient care devices. Patient care devices include: Blood Pressure Cuffs, Stethoscopes, Thermometers, Glucometers, Otoscopes, O2 Sats, and Stretchers etc. The contaminated devices should be cleaned in the dirty zone. Cleaning removes soil and body materials (e.g. blood, organic soils) and must occur as an integral first step before. 1. Gather all equipment, cleaning solutions and materials required to clean the patient care devices. 2. WASH hands and put gloves prior to cleaning the devices. Personal protective equipment should be changed if torn or soiled. 3. Visible or gross soil present and/or blood or body fluid spills must be removed prior to cleaning. [See Protocol for Cleaning & Disinfecting a Blood or Body Fluid spill.]
3 4. Clean all surfaces of the patient care equipment or devices. Where appropriate, dismantle the devices to ensure that all surfaces can be cleaned using the AHP Solution. To ensure that cross contamination does not occur use clean cloths for each device to be cleaned. If using an open bucket system, ensure that solutions do not become contaminated (NO DOUBLE DIPPING). Allow surfaces to remain wet for 30 seconds to achieve the 30-second Broad-Spectrum Sanitizing claim. 5. To disinfect all surfaces of the patient care devices, reapply the AHP Solution and allow surfaces to remain wet for 5-minutes to achieve the Bactericidal, General Virucide, Fungicidal and Tuberculocidal claim. 6. Soiled rags should be placed in a bag for laundering. Disposable cloths should be disposed as regular waste in garbage bags. 7. Remove and discard gloves, WASH hands. SUMMARY OF PROCEDURES FOR SEMI-CRITICAL DEVICES Semi-critical disinfection involves disinfection of items that come in contact with mucous membranes and intact skin, but not with internal, natural sterile areas of the body. Cleaning of semi-critical items/equipment is an important step in the disinfection process to ensure the disinfecting of the items/equipment will be successful. Cleaning must be thoroughly done before processing because organic material may protect microorganisms from the disinfection process. Recommended Procedures for Cleaning and Disinfecting of Semi-Critical Devices 1. Cleaning should take place between each device/equipment usage. Items will be disassembled (as appropriate) and thoroughly cleaned. 2. Semi-critical items may be contaminated with dried or wet sputum and/or blood and should be cleaned using a detergent such as Accel Wash (Diluted 1:128 or 32 ml Accel Wash Concentrate to 4 Litres water), rinsed, and dried prior to using PREVention for High Level Disinfection. 3. High Level Disinfection consists of immersing the device/equipment in the PREVention solution for 5-minutes. 4. Once the 5-minute High Level Disinfection contact time has passed, the device/equipment should be removed from the PREVention solution using clean, disinfected tongs or forceps. 5. The disinfected device/equipment should be thoroughly rinsed using sterile water when practical, otherwise potable water is acceptable for semi-critical devices not intended for use on immunocompromised patients or potentially immunocompromised patients based on institutional procedures (ie. high-risk populations). 6. Following removal from PREVention solution, thoroughly rinse device/equipment by immersing it completely in a large volume of water keeping the device/equipment totally immersed for a minimum of 1-minute repeating this step for 3 consecutive times. 7. Manually flush all lumens. 8. Remove device/equipment and discard the rinse water. Always use fresh water for each rinse. Do not reuse the water for rinsing or any other purposes. 9. Following the rinsing step the device/equipment should be dried and stored in a suitable container (ie. clear polyethylene bag) for future use.
4 SUMMARY OF PROCEDURES FOR CRITICAL DEVICES Critical instruments are those items that enter sterile tissues, including the vascular system and present a high risk of infection if the item is contaminated with any microorganisms, including bacterial spores. Reprocessing critical items involves meticulous cleaning followed by sterilization. Cleaning of critical items/equipment is an important step in the disinfection process to ensure the disinfecting of the items/equipment will be successful. Cleaning must be thoroughly done before processing because organic material may protect microorganisms from the disinfection process. Recommended Procedures for Cleaning and Disinfecting Critical Devices 1. Cleaning should take place between each device/equipment usage. Items will be disassembled (as appropriate) and thoroughly cleaned. 2. Critical items may be contaminated with dried or wet sputum and/or blood and should be cleaned using a detergent such as Accel Wash (Diluted 1:128 or 32 ml Accel Wash Concentrate to 4 Litres water), rinsed, and dried prior to using Accel CS20 for Chemical Sterilization. 3. Chemical Sterilization consists of immersing the device/equipment in the Accel CS20 solution for 20-minutes. 4. Once the 20-minute Chemical Sterilization contact time has passed, the device/equipment should be removed from the Accel CS20 solution using clean, disinfected tongs or forceps. 5. The disinfected device/equipment should be thoroughly rinsed using sterile water. 6. Following removal from Accel CS20 solution, thoroughly rinse device/equipment by immersing it completely in a large volume of water keeping the device/equipment totally immersed for a minimum of 1-minute repeating this step for 3 consecutive times. 7. Manually flush all lumens. 8. Remove device/equipment and discard the rinse water. Always use fresh water for each rinse. Do not reuse the water for rinsing or any other purposes. 9. Following the rinsing step the device/equipment should be dried and stored in a suitable container (ie. clear polyethylene bag) for future use. Recommended Procedures for Cleaning & Disinfecting of Blood & Body Fluid Spills Appropriate personal protective equipment should be worn for cleaning up a body fluid spill. Gloves should be worn during the cleaning and disinfecting procedures. If the possibility of splashing exists, the worker should wear a face shield and gown. For large spills, overalls, gowns or aprons as well as boots or protective shoe covers should be worn. Personal protective equipment should be changed if torn or soiled, and always removed before leaving the location of the spill, and then wash hands. 1. WASH hands and put on gloves. 2. If the possibility of splashing exists, the worker should wear a face shield and gown. For large spills, overalls, gowns or aprons as well as boots or protective shoe covers should be worn. Personal protective equipment should be changed if torn or soiled and always removed before leaving the location of the spill. 3. Apply the AHP Solution to spill wait 30 seconds.
5 4. Blot up the blood with disposable towels. Dispose of paper towel in plastic-lined waste receptacle. 5. Spray or wipe surface with the AHP Solution wait 5 minutes. Wipe dry with disposable paper towel. Discard paper towel as above. 6. Remove gloves and dispose in plastic-lined waste receptacle. 7. WASH hands. Disposal of Infectious Material All cleaning cloths gloves and handled tools used for the decontamination of a suspected Avian Flu virus case must be placed in a clearly marked plastic lined waste receptacle. Decontaminate all wastes before disposal; steam sterilization, chemical disinfection and or incineration. Instructions for Confirmatory Testing of 7% AHP Concentrate Surface Disinfectants The Accelerated Hydrogen Peroxide Test Strip (Part No. AHP500) can be used for confirmatory testing when required by facility protocol. These strips are easy to use dip-and-read reagents strips for a pass or fail determination of the hydrogen peroxide concentration in the 7% AHP Concentrate Surface Disinfectant solution. 1. Remove a test strip and immediately close the container. 2. Dip the test strip into the Diluted AHP solution to be tested for 1-second ensuring that the reaction zone is completely wetted. 3. Remove the test strip and shake of excess liquid. 4. Wait for 120-seconds then compare the reaction zone with the colour scale. NOTE: The purpose of confirmatory testing is not to extend the shelf life beyond the 30-day claim. Should the test strip show that the Diluted AHP Solution still meets the targeted level of hydrogen peroxide after 30 days the product MUST still be disposed to ensure compliance with testing and label claims. Instructions for Confirmatory Testing of PREVention (2% AHP) The Accelerated Hydrogen Peroxide Test Strip 2% (Part No. AHP2) should be used for confirmatory testing. These strips are easy to use dip-and-read reagents strips for a pass or fail determination of the hydrogen peroxide concentration in the PREVention solution. 1. Remove a test strip and immediately close the container. 2. Dip the test strip into the PREVention solution to be tested for 1-second ensuring that the reaction zone is completely wetted. 3. Remove the test strip and shake off excess liquid. 4. Wait for 60-seconds then compare the reaction zone with the colour scale. NOTE: The purpose of confirmatory testing is not to extend the reuse life beyond the 14-day claim. Should the test strip show that the PREVention solution still meets the targeted level of hydrogen peroxide the product MUST still be disposed. Instructions for Confirmatory Testing of Accel CS20 (7% AHP)
6 The Accelerated Hydrogen Peroxide Test Strip 7% (Part No. AHP7) should be used for confirmatory testing. These strips are easy to use dip-and-read reagents strips for a pass or fail determination of the hydrogen peroxide concentration in the PREVention solution. 1. Remove a test strip and immediately close the container. 2. Dip the test strip into the Accel CS20 solution to be tested for 1-second ensuring that the reaction zone is completely wetted. 3. Remove the test strip and shake off excess liquid. 4. Wait for 35-seconds then compare the reaction zone with the colour scale. NOTE: The purpose of confirmatory testing is not to extend the reuse life beyond the 14-day claim. Should the test strip show that the Accel CS20 solution still meets the targeted level of hydrogen peroxide the product MUST still be disposed. References: Provincial Infectious Diseases Advisory Committee, Best Practices for Cleaning, Disinfection and Sterilization in All Healthcare Settings, 2006 Public Health Agency of Canada, Infection Control Guidelines for Hand Washing, Cleaning, Disinfection and Sterilization in Healthcare, Volume 24S8, 1998
Technical Information. Clorox Healthcare Bleach Germicidal Cleaner OVERVIEW. Clorox Healthcare Bleach Germicidal
 OVERVIEW Bleach Germicidal Cleaner is engineered as a ready-to-use sporicidal cleaner-disinfectant system for healthcare facilities. This EPA-registered disinfectant contains sodium hypochlorite and other
OVERVIEW Bleach Germicidal Cleaner is engineered as a ready-to-use sporicidal cleaner-disinfectant system for healthcare facilities. This EPA-registered disinfectant contains sodium hypochlorite and other
Disinfectants in Personal Services Settings
 Disinfectants in Personal Services Settings 2014 Ontario Branch Canadian Institute of Public Health Inspectors (CIPHI) Conference Cecilia Alterman, MEd, BES, BASc, CPHI (C) (A) Manager, Control of Infectious
Disinfectants in Personal Services Settings 2014 Ontario Branch Canadian Institute of Public Health Inspectors (CIPHI) Conference Cecilia Alterman, MEd, BES, BASc, CPHI (C) (A) Manager, Control of Infectious
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions
 Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Policy: C-08-PRO Reviewed: 7/08
 Written: 1979 Policy: Reviewed: 7/08 Procedure Policy Manual Revised: 81, 84, 85, 86, 87, 88, 89, 90, 92, 93, 95 Ambulatory Care Division 2/97, 7/98, 7/00, 7/02, 7/04, 7/06, 2/09, 7/10 LSU Health Sciences
Written: 1979 Policy: Reviewed: 7/08 Procedure Policy Manual Revised: 81, 84, 85, 86, 87, 88, 89, 90, 92, 93, 95 Ambulatory Care Division 2/97, 7/98, 7/00, 7/02, 7/04, 7/06, 2/09, 7/10 LSU Health Sciences
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER SANITARY REQUIREMENTS TABLE OF CONTENTS
 RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER 0200-03 SANITARY REQUIREMENTS TABLE OF CONTENTS 0200-03-.01 Applicability 0200-03-.02 Violations 0200-03-.03 Location 0200-03-.04 Communicable
RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER 0200-03 SANITARY REQUIREMENTS TABLE OF CONTENTS 0200-03-.01 Applicability 0200-03-.02 Violations 0200-03-.03 Location 0200-03-.04 Communicable
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
What is infection control?
 Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection Control Solutions Product Solutions. for Surfaces & Skin
 Infection Control Solutions Product Solutions for Surfaces & Skin Hand Care Surface Disinfection Skin Antisepsis UV Technology Hand Hygiene AloeGuard Antimicrobial Soap GBG AloeGel Instant Hand Sanitizer
Infection Control Solutions Product Solutions for Surfaces & Skin Hand Care Surface Disinfection Skin Antisepsis UV Technology Hand Hygiene AloeGuard Antimicrobial Soap GBG AloeGel Instant Hand Sanitizer
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Nova Scotia Safe Body Art Standards
 Nova Scotia Safe Body Art Standards Crown copyright, Province of Nova Scotia, 2018 Nova Scotia Safe Body Art Standards Environment, December 2018. ISBN 978-1-55457-905-1 Standard: Nova Scotia Safe Body
Nova Scotia Safe Body Art Standards Crown copyright, Province of Nova Scotia, 2018 Nova Scotia Safe Body Art Standards Environment, December 2018. ISBN 978-1-55457-905-1 Standard: Nova Scotia Safe Body
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
Table 6: Detailed Infection Prevention and Control Procedures for Tattooing and Micropigmentation. Use During Tattooing
 FACT SHEET Table 6: Detailed Infection Prevention and Control Procedures for and Micropigmentation 1. Skin Preparation Spray bottle with a solution of soap and water Single use disposable razor The skin
FACT SHEET Table 6: Detailed Infection Prevention and Control Procedures for and Micropigmentation 1. Skin Preparation Spray bottle with a solution of soap and water Single use disposable razor The skin
BTB000 Manufacturers Product Code: TAB/R/DR
 BTB000 Manufacturers Product Code: TAB/R/DR Red Polythene Drug Round Tabard (21 x 24 / 30mu) black print Drug Round in Progress. One size fits all. Drug round tabards improve the efficiency and accuracy
BTB000 Manufacturers Product Code: TAB/R/DR Red Polythene Drug Round Tabard (21 x 24 / 30mu) black print Drug Round in Progress. One size fits all. Drug round tabards improve the efficiency and accuracy
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
RULES OF TENNESSEE STATE BOARD OF COSMETOLOGY CHAPTER SANITARY RULES TABLE OF CONTENTS
 RULES OF TENNESSEE STATE BOARD OF COSMETOLOGY CHAPTER 0440-2 SANITARY RULES TABLE OF CONTENTS 0440-2-.01 Definitions 0440-2-.10 Animals 0440-2-.02 Applicability 0440-2-.11 High Frequency Electric Current
RULES OF TENNESSEE STATE BOARD OF COSMETOLOGY CHAPTER 0440-2 SANITARY RULES TABLE OF CONTENTS 0440-2-.01 Definitions 0440-2-.10 Animals 0440-2-.02 Applicability 0440-2-.11 High Frequency Electric Current
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
under Council Medical Devices Directive (93/42/EEC) as updated directive 2007/47/EEC
 Page 1 of 7 under Council Medical Devices Directive (93/42/EEC) as updated directive 2007/47/EEC New Miana Pura East, Roras Road, Sialkot - Pakistan Tel: +92-52-3560135 Fax: +92-52-3563647 E-mail: info@longstoneintl.com
Page 1 of 7 under Council Medical Devices Directive (93/42/EEC) as updated directive 2007/47/EEC New Miana Pura East, Roras Road, Sialkot - Pakistan Tel: +92-52-3560135 Fax: +92-52-3563647 E-mail: info@longstoneintl.com
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
The Importance of Equipment and Surface Cleaning/Disinfection. Training material developed in collaboration with:
 The Importance of Equipment and Surface Cleaning/Disinfection Training material developed in collaboration with: Objectives Identify which clinic surfaces are vulnerable to contamination Identify contact
The Importance of Equipment and Surface Cleaning/Disinfection Training material developed in collaboration with: Objectives Identify which clinic surfaces are vulnerable to contamination Identify contact
ABOUT US. Behban pharmed lotus co. is a leading supplier. We have valid international certificates such
 ABOUT US Behban pharmed lotus co. is a leading supplier of products and technologies for cleaning and disinfection. We develop, produce and market for professional applications in hospital hygiene and
ABOUT US Behban pharmed lotus co. is a leading supplier of products and technologies for cleaning and disinfection. We develop, produce and market for professional applications in hospital hygiene and
SUBCHAPTER 14H - SANITATION SECTION SANITATION
 SUBCHAPTER 14H - SANITATION SECTION.0100 - SANITATION 21 NCAC 14H.0101 COPY OF RULES TO COSMETOLOGY STUDENTS Cosmetic art schools shall give a copy of the sanitation rules governing the practice of the
SUBCHAPTER 14H - SANITATION SECTION.0100 - SANITATION 21 NCAC 14H.0101 COPY OF RULES TO COSMETOLOGY STUDENTS Cosmetic art schools shall give a copy of the sanitation rules governing the practice of the
Infection Prevention and Control Best Practices for Personal Services Settings
 Infection Prevention and Control Best Practices for Personal Services Settings Infection Prevention and Control Unit Public Health Division Ministry of Health and Long-Term Care January 2009 Table of Contents
Infection Prevention and Control Best Practices for Personal Services Settings Infection Prevention and Control Unit Public Health Division Ministry of Health and Long-Term Care January 2009 Table of Contents
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
rooo.lb IOWA COUNTY ORDINANCE NO TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS:
 .. rooo.lb IOWA COUNTY ORDINANCE NO. 4-196 TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS: SECTION I: The following ordinance of Iowa County, Wisconsin is hereby created
.. rooo.lb IOWA COUNTY ORDINANCE NO. 4-196 TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS: SECTION I: The following ordinance of Iowa County, Wisconsin is hereby created
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Current Status: Active PolicyStat ID: Original Policy: 10/1986 Last Reviewed: 01/2016 Last Revised: 01/2016 Next Review: 01/2019
 Current Status: Active PolicyStat ID: 2085666 Original Policy: 10/1986 Last Reviewed: 01/2016 Last Revised: 01/2016 Next Review: 01/2019 Owner: Policy Area: References: Applicability: Tracy Dodson: Director
Current Status: Active PolicyStat ID: 2085666 Original Policy: 10/1986 Last Reviewed: 01/2016 Last Revised: 01/2016 Next Review: 01/2019 Owner: Policy Area: References: Applicability: Tracy Dodson: Director
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
Hand Hygiene. Policy Title: Hand Hygiene Policy Number: 05. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
WHISTON WORRYGOOSE JUNIOR AND INFANT SCHOOL
 WHISTON WORRYGOOSE JUNIOR AND INFANT SCHOOL Part of White Woods Academy Trust BODILY FLUID HYGIENE POLICY Approved by Governors: September 2017 Review Date: September 2019 Statement of intent At Whiston
WHISTON WORRYGOOSE JUNIOR AND INFANT SCHOOL Part of White Woods Academy Trust BODILY FLUID HYGIENE POLICY Approved by Governors: September 2017 Review Date: September 2019 Statement of intent At Whiston
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Hygienic requirements for tattoo and piercing studios
 Hygienic requirements for tattoo and piercing studios Activities injuring the skin or mucus membrane are linked to an increased infection risk for diseases transferred by blood and serum. To avoid transferable
Hygienic requirements for tattoo and piercing studios Activities injuring the skin or mucus membrane are linked to an increased infection risk for diseases transferred by blood and serum. To avoid transferable
Instructor Guide. Georgia Department of Technical and Adult Education. Decontamination and Infection Control
 Instructor Guide Georgia Department of Technical and Adult Education Decontamination and Infection Control Copyright October 2002 by. All rights reserved. No part of this manual may be reproduced or transmitted
Instructor Guide Georgia Department of Technical and Adult Education Decontamination and Infection Control Copyright October 2002 by. All rights reserved. No part of this manual may be reproduced or transmitted
Clinical Environments Cleaning, Care, and Maintenance
 Clinical Environments Cleaning, Care, and Maintenance With regular care and maintenance, your Herman Miller clinical products will provide many years of superior performance and satisfaction in high traffic,
Clinical Environments Cleaning, Care, and Maintenance With regular care and maintenance, your Herman Miller clinical products will provide many years of superior performance and satisfaction in high traffic,
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
PROFESSIONAL PRODUCTS FOR YOUR HYGIENE AND HEALTH
 PROFESSIONAL PRODUCTS FOR YOUR HYGIENE AND HEALTH ABOUT US Behban pharmed lotus co. is a leading supplier of products and technologies for cleaning and disinfection. We develop, produce and market for
PROFESSIONAL PRODUCTS FOR YOUR HYGIENE AND HEALTH ABOUT US Behban pharmed lotus co. is a leading supplier of products and technologies for cleaning and disinfection. We develop, produce and market for
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Surgical Hand Scrub Updates. Surgical Hand Scrub Updates
 Surgical Hand Scrub Updates Surgical Hand Scrub Updates February 2009 Objectives Review facts on Pathogens Review AORN and CDC guidelines for hand scrubs Review steps to Water-based hand scrub application
Surgical Hand Scrub Updates Surgical Hand Scrub Updates February 2009 Objectives Review facts on Pathogens Review AORN and CDC guidelines for hand scrubs Review steps to Water-based hand scrub application
We understand that a competitor has raised the following issues which we will address in this letter.
 March 01, 2010 Dear Customer, Thank you for your recent inquiry into PURELL Waterless Surgical Scrub. PURELL is an effective surgical scrub formulation that meets the requirements of the surgical scrub
March 01, 2010 Dear Customer, Thank you for your recent inquiry into PURELL Waterless Surgical Scrub. PURELL is an effective surgical scrub formulation that meets the requirements of the surgical scrub
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control
 The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control Author University Health Service, The University of Hong Kong Approved By Task Force on Infectious Diseases,
The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control Author University Health Service, The University of Hong Kong Approved By Task Force on Infectious Diseases,
Welcome. to Infection Control. Cutan. Trada.Co
 Trada.Co Welcome to Infection Control Deb Ltd, the makers of the Cutan Hand Hygiene range, one of the world s leading manufacturers of occupational skin care products, with over 60 years experience. In
Trada.Co Welcome to Infection Control Deb Ltd, the makers of the Cutan Hand Hygiene range, one of the world s leading manufacturers of occupational skin care products, with over 60 years experience. In
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
INFECTION PREVENTION STANDARDS FOR THE PRACTICE OF ELECTROLYSIS (Rev. MAY 2016) PREFACE
 INFECTION PREVENTION STANDARDS FOR THE PRACTICE OF ELECTROLYSIS (Rev. MAY 2016) PREFACE The American Electrology Association has adopted these Infection Prevention Standards for the Practice of Electrolysis
INFECTION PREVENTION STANDARDS FOR THE PRACTICE OF ELECTROLYSIS (Rev. MAY 2016) PREFACE The American Electrology Association has adopted these Infection Prevention Standards for the Practice of Electrolysis
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
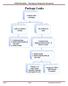 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
ALVERNIA UNIVERSITY MRSA CLEANING AND DISINFECTION PROCEDURES REVISION: EFFECTIVE
 : EFFECTIVE DATE: 4/2/204 A. SUMMARY MRSA is methicillin-resistant Staphylococcus aureus, a type of staph bacteria that is resistant to certain antibiotics and may cause skin and other infections. You
: EFFECTIVE DATE: 4/2/204 A. SUMMARY MRSA is methicillin-resistant Staphylococcus aureus, a type of staph bacteria that is resistant to certain antibiotics and may cause skin and other infections. You
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Hand Hygiene ORGANIZATIONAL: Affects two or more departments.
 Hand Hygiene ORGANIZATIONAL: Affects two or more departments. Folder Infection Prevention Sub-Folder Original 1/1/1987 Scope All Effective Date Approved (Approver/Date) Last Reviewed/ Revised Date IPC:
Hand Hygiene ORGANIZATIONAL: Affects two or more departments. Folder Infection Prevention Sub-Folder Original 1/1/1987 Scope All Effective Date Approved (Approver/Date) Last Reviewed/ Revised Date IPC:
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
The OZAKI VRec Sizer (13, 15, 17 mm) JD-001-P FOR The OZAKI VRec Sizer System INSTRUCTIONS FOR USE
 The OZAKI VRec Sizer (13, 15, 17 mm) JD-001-P FOR The OZAKI VRec Sizer System INSTRUCTIONS FOR USE The OZAKI VRec Sizer (Ref: JD-001-P) Rx Only CAUTION: Federal law (USA) restricts this device to sale
The OZAKI VRec Sizer (13, 15, 17 mm) JD-001-P FOR The OZAKI VRec Sizer System INSTRUCTIONS FOR USE The OZAKI VRec Sizer (Ref: JD-001-P) Rx Only CAUTION: Federal law (USA) restricts this device to sale
City and County of Denver Rules and Regulations for Body Artist, Body Art Establishments, and Mobile Body Art Vehicles Chapter 24 DRMC
 City and County of Denver Rules and Regulations for Body Artist Body Art Establishments and Mobile Body Art Vehicles Chapter 24 DRMC Adopted by the Board of Environmental Health on March 11 1999 And Amended
City and County of Denver Rules and Regulations for Body Artist Body Art Establishments and Mobile Body Art Vehicles Chapter 24 DRMC Adopted by the Board of Environmental Health on March 11 1999 And Amended
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
CHAPTER 64E-19 BODY PIERCING
 CHAPTER 64E-19 BODY PIERCING 64E-19.001 64E-19.002 64E-19.003 64E-19.004 64E-19.005 64E-19.006 64E-19.007 64E-19.008 General Definitions Forms Requirements for Premises Requirements for Sterilizing Jewelry
CHAPTER 64E-19 BODY PIERCING 64E-19.001 64E-19.002 64E-19.003 64E-19.004 64E-19.005 64E-19.006 64E-19.007 64E-19.008 General Definitions Forms Requirements for Premises Requirements for Sterilizing Jewelry
DEPARTMENT OF LABOR, LICENSING AND REGULATION BOARD OF COSMETOLOGY
 Agency Name: Board of Cosmetology - Labor, Licensing and Regulation Statutory Authority: 40-1-70 and 40-13-80 Document Number: 4720 Proposed in State Register Volume and Issue: 40/10 House Committee: Regulations
Agency Name: Board of Cosmetology - Labor, Licensing and Regulation Statutory Authority: 40-1-70 and 40-13-80 Document Number: 4720 Proposed in State Register Volume and Issue: 40/10 House Committee: Regulations
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Infection prevention. Infection prevention. FoamING sanitizer Liquid sanitizer Foaming soap Lotion soap Lotion. EB LI, (Rev.
 Infection prevention. Infection prevention. FoamING sanitizer Liquid sanitizer Foaming soap Lotion soap Lotion EB-95720-LI, (Rev. 1-11-10) Now, it doesn t have to be, with NEW A complete line of hand hygiene
Infection prevention. Infection prevention. FoamING sanitizer Liquid sanitizer Foaming soap Lotion soap Lotion EB-95720-LI, (Rev. 1-11-10) Now, it doesn t have to be, with NEW A complete line of hand hygiene
APPROVAL REVIEW PROCEDURES
 Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
The future of disinfection is here
 The future of disinfection is here A breakthrough line of EPA-registered disinfectants from Clorox harnesses the power of hydrogen peroxide for disinfecting and exceptional cleaning performance. Now you
The future of disinfection is here A breakthrough line of EPA-registered disinfectants from Clorox harnesses the power of hydrogen peroxide for disinfecting and exceptional cleaning performance. Now you
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
Infection Control Product Catalogue 2013
 Infection Control Product Catalogue 2013 protect.co.uk Sanitas Healthcare distributes its range of infection control products for a wide range of clients including the UK s National Health Service (NHS),
Infection Control Product Catalogue 2013 protect.co.uk Sanitas Healthcare distributes its range of infection control products for a wide range of clients including the UK s National Health Service (NHS),
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Aseptic Milk Sampling for Bacteriology
 Aseptic Milk Sampling for Bacteriology Year Group: BVSc1 + Document number: CSL_F02 Equipment for this station: Equipment list: Cow model (fitted with milking udder) Wet wipes Paper towel Teat dip bottle
Aseptic Milk Sampling for Bacteriology Year Group: BVSc1 + Document number: CSL_F02 Equipment for this station: Equipment list: Cow model (fitted with milking udder) Wet wipes Paper towel Teat dip bottle
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
(c) BODY ART ESTABLISHMENT means any location, whether temporary or permanent, where the practices of body art are performed.
 DEPARTMENT OF PUBLIC HEALTH AND ENVIRONMENT Division of Environmental Health and Sustainability BODY ART ESTABLISHMENTS 6 CCR 1010-22 [Editor s Notes follow the text of the rules at the end of this CCR
DEPARTMENT OF PUBLIC HEALTH AND ENVIRONMENT Division of Environmental Health and Sustainability BODY ART ESTABLISHMENTS 6 CCR 1010-22 [Editor s Notes follow the text of the rules at the end of this CCR
Ventricular Assist Device (VAD) Exit Site Care Guidelines
 Ventricular Assist Device (VAD) Exit Site Care Guidelines I. STATEMENT OF PURPOSE The Ventricular Assist Device (VAD) Exit Site Care Guidelines are intended to provide standardization and continuity of
Ventricular Assist Device (VAD) Exit Site Care Guidelines I. STATEMENT OF PURPOSE The Ventricular Assist Device (VAD) Exit Site Care Guidelines are intended to provide standardization and continuity of
UniTab. Disinfectant & Sanitizing Tablets A bleach alternative in tablet form DIN: Code: (120 x 6.55 g tablet)
 UniTab Disinfectant & Sanitizing Tablets A bleach alternative in tablet form Code: 53379 (120 x 6.55 g tablet) DIN: 02470381 A bit about bleach... Bleach is one of the most commonly used and relied upon
UniTab Disinfectant & Sanitizing Tablets A bleach alternative in tablet form Code: 53379 (120 x 6.55 g tablet) DIN: 02470381 A bit about bleach... Bleach is one of the most commonly used and relied upon
RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER SANITARY RULES TABLE OF CONTENTS
 RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER 0440-02 SANITARY RULES TABLE OF CONTENTS 0440-02-.01 Definitions 0440-02-.10 Animals 0440-02-.02 Applicability 0440-02-.11 High Frequency
RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER 0440-02 SANITARY RULES TABLE OF CONTENTS 0440-02-.01 Definitions 0440-02-.10 Animals 0440-02-.02 Applicability 0440-02-.11 High Frequency
CLINITEX A RANGE OF WIPES, CLEANSING & JANITORIAL PRODUCTS FOR HEALTHCARE PROFESSIONALS
 Clinitex Professional Care Products Manufactured by Technical Textile Services Ltd. (Techtex) Units 7 & 8, Rhodes Business Park, Silburn Way, Middleton, Manchester, M24 4NE, UK t: +44 (0)161 643 3000 f:
Clinitex Professional Care Products Manufactured by Technical Textile Services Ltd. (Techtex) Units 7 & 8, Rhodes Business Park, Silburn Way, Middleton, Manchester, M24 4NE, UK t: +44 (0)161 643 3000 f:
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Table 5: Detailed Infection Prevention and Control Procedures for Body Piercing. drape the piercing site.
 FACT SHEET Table 5: Detailed Infection Prevention and Control Procedures for Body Piercing Equipment / Single use towel 1. Client preparation A towel may be used to drape the piercing site. The towel should
FACT SHEET Table 5: Detailed Infection Prevention and Control Procedures for Body Piercing Equipment / Single use towel 1. Client preparation A towel may be used to drape the piercing site. The towel should
Clorox Total 360 System
 Electrostatic Technology The Clorox Total 360 System has proven electrostatic sprayer technology with wraparound coverage. Electrostatic technology enables Clorox sanitizers and disinfectants to reach
Electrostatic Technology The Clorox Total 360 System has proven electrostatic sprayer technology with wraparound coverage. Electrostatic technology enables Clorox sanitizers and disinfectants to reach
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Instrument Cleaning. Dental Clinics
 1 CLEANING FOR INFECTION CONTROL Dental Clinics 2 This presentation is designed to reflect the recommendations given in the following Australian Standards and Guidelines. Throughout this discussion we
1 CLEANING FOR INFECTION CONTROL Dental Clinics 2 This presentation is designed to reflect the recommendations given in the following Australian Standards and Guidelines. Throughout this discussion we
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
