(12) United States Patent (10) Patent No.: US 7,754,774 B2
|
|
|
- Margery Lindsey Crawford
- 5 years ago
- Views:
Transcription
1 USOO B2 (12) United States Patent (10) Patent No.: Kobayashi et al. (45) Date of Patent: Jul. 13, 2010 (54) ANTISEPTIC BACTERICIDES AND 2004/O A1 7/2004 Ono et al , COSMETICS, DRUGS AND FOODS CONTAINING THE ANTISEPTC FOREIGN PATENT DOCUMENTS BACTERICDES (75) Inventors: Aki Kobayashi, Kyoto (JP); Hiroya Okamoto, Osaka (JP); Fumihiro Okada, Osaka (JP) (73) Assignee: Mandom Corporation, Osaka (JP) (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 U.S.C. 154(b) by 115 days. (21) Appl. No.: 10/500,358 (22) PCT Filed: Sep. 25, 2003 (86). PCT No.: S371 (c)(1), (2), (4) Date: PCT/UP03/12288 Jun. 30, 2004 (87) PCT Pub. No.: WO2004/ PCT Pub. Date: Apr. 8, 2004 (65) Prior Publication Data US 2005/ A1 May 19, 2005 (30) Foreign Application Priority Data Sep. 26, 2002 (JP) (51) Int. Cl. AOIN3L/00 ( ) A6 IK3I/045 ( ) B65B 55/00 ( ) A2.3L 3/36 ( ) A23B 4/20 ( ) (52) U.S. Cl /738: 514/724; 426/326 (58) Field of Classification Search /404; 510/131; 514/738,724; 426/326 See application file for complete search history. (56) References Cited U.S. PATENT DOCUMENTS 2,550,255 A * 4, 1951 Jensen ,332 5,403,587 A * 4, 1995 McCue et al ,736 5,630,847 A * 5/1997 Roetker... 8,137 6,348,187 B1 * 2/2002 Pan et al / / A1* 11/2001 Clarkson et al ,564 DE SO3 A1 4/2001 EP 5870O2 3, 1994 EP O A1 5, 1998 JP , 1984 JP A 10, 1992 JP , 1993 JP , 1998 JP , 1999 JP , 1999 JP , 1999 JP O 2, 2001 JP , 2001 JP A 10, 2001 JP T 2002 JP , 2003 JP , 2003 JP A 1, 2004 OTHER PUBLICATIONS Jeongmok Kim et al., Antibacterial Activity of Some Essential Oil Components against Five Foodborne Pathogens. J. Agric. Food Chem., 1995, vol.43, pp Kurita et al., Synergistic Antimicrobial Effect of Sodium Chloride and Essential Oil Components'. Agricultural and Biological Chem istry, vol. 46, No. 1, 1982, pp XP Professor Dr. Hildebert Wagner, Pharmazeutische Biologie Drogen und ihre Inhaltsstoffe Gustav Fischer Verlag, Stuttgart, 1988 XPOO Supplementary European Search Report dated Nov. 20, * cited by examiner Primary Examiner Yong S Chong (74) Attorney, Agent, or Firm Westerman, Hattori, Daniels & Adrian, LLP. (57) ABSTRACT The purpose of the present invention is to provide antiseptic disinfectant, and cosmetics and toiletries, medicine or food containing the same, which enhance the antibacterial activity that 1.2-alkanediol originally have against a broad range of strains by compounding 1.2-alkanediol with 5-10 carbons and a certain fragrance component. The present invention relates to an antiseptic disinfectant, and cosmetics and toiletries, medicine or food containing the same, which include 1,2-alkanediol with 5-10 carbons, and one or more materials selected from a group of thymol. eugenol, citronellal, terpinyl acetate, citronellol and B-pinene. 4 Claims, 8 Drawing Sheets
2 U.S. Patent Jul. 13, 2010 Sheet 1 of 8 FIG.1 MIC in changing the rate of material B
3 U.S. Patent Jul. 13, 2010 Sheet 2 of 8 FIG.2 (a) MIC as to C. albicans (u g/ml) (b) MIC as to S.aureus (u g/ml) O 500 OOO SCO 2000 O SOO OOO SOO 2 OOO 25OO 1,2-octanediol (c) MIC as to Paeruginosa ( u g/ml) 3OOO " OOO 500 O O COO 2OOO OOO 4OOO
4 U.S. Patent Jul. 13, 2010 Sheet 3 of 8 FIG.3 (a) MIC as to C. albicans ( u g/ml) (b) MIC as to S. aureus (u g/ml) re g OO s OOO O O SOO OOO SOO l.2 octanediol 1,2-octanediol
5 U.S. Patent Jul. 13, 2010 Sheet 4 of 8 FIG.4 MIC as to C. albicans (u g/ml) (b) MIC as to S. aureus (u g/ml) O l,2'octanediol O 500 OOO 500 2OOO 250C FIG MIC as to Calbicans (u g/ml) (b) 8000 MIC as to S.aureus ( u g/ml) OOO 0 SOO OOO O 500 OOO ,2'octanediol
6 U.S. Patent Jul. 13, 2010 Sheet 5 of 8 FIG (b) MIC as to S. aureus (11 g/ml) O 500 OOO O SOO OOO SOO ,2'octanediol l, 2 octanediol (C) 6000 MIC as to E.coli (u g/ml) OOO O OOO 1500 l, 2 octanediol
7 U.S. Patent Jul. 13, 2010 Sheet 6 of 8 FIG.7 (a) ( b) MIC as to S. aureus (u g/ml) a 2SOOO OOOO OOOO U 5000 d a OOOO S r O a 4000 O O O SOO OOO O 500 OOO OO ( C) MIC as to E.coli (u g/ml) gu 25OOO d S 9, SOOO O 500 1OOO SOO
8 U.S. Patent Jul. 13, 2010 Sheet 7 of 8 FIG.8 (a) MIC as to C. albicans (u g/ml) (b ) MIC as to S. aureus (u g/ml) 25 OOOOO OOOOO OOOOO O O l,2-octanediol O (C) MIC as to Paeruginosa ( u g/ml) (d ) MIC as to E.coli (u g/ml) O OOO 2OOO 3OOO 4000 l, 2 octanediol O 500 OOO 500 l, 2 octanediol
9 U.S. Patent Jul. 13, 2010 Sheet 8 of 8 FIG.9 MIC as to C.albicans (u g/ml) MIC as to S.aureus (u g/ml) OOO O SOO l,2-octanediol MIC as to Paeruginosa (u g/ml) ( d ) 6000 MIC as to E.coli (u g/ml) OOO OOO O OOO 2OOO 3000 O SOO
10 1. ANTISEPTIC BACTERICDES AND COSMETICS, DRUGS AND FOODS CONTAINING THE ANTISEPTC BACTERICDES TECHNICAL FIELD The present invention relates to an antiseptic disinfectant, and cosmetics and toiletries, medicine or food containing the same. The purpose of the present invention is to provide the antiseptic disinfectant, and cosmetics and toiletries, medicine or food containing the same which enhance the antibacterial activity that 1.2-alkanediol originally have against a broad range of Strains by compounding 1,2-alkanediol with 5-10 carbons and a certain fragrance component. TECHNICAL BACKGROUND Paraben, benzoic acid, Salicylic acid and so on have been used for the antiseptic disinfectants in the cosmetics and toiletries (including quasi drugs), medicine or food. As the above-mentioned conventional antiseptic disinfectants have low degree in their safety because of the high skin irriant etc., they have a defect that the range of the concentration for use tends to be limited. For example, the restricted concentration of paraben and benzoate for use is 1%, and the restricted concentration of benzoic acid and Salicylic acid for use is 0.2%. Further, there have been problems that the antiseptic disinfection effect of those antiseptic disinfectants is not reli able because they are easily influenced by ph, and that anti septic and antibacterial activity may remarkably fall by the combination use with other compounds such as surfactant. Still further, the number of people who are allergic to those antiseptic disinfectants is increasing in recent years. There fore, more people are oriented to the safety, and so the demand for the cosmetics and toiletries, medicine and food without the antiseptic disinfectant or containing very reduced amount of it is increasing. As the technical means to reduce or remove the antiseptic disinfectant, the antiseptic disinfectant containing 1,2-al kanediol (referring to publication No of Pub lished Japanese patent application) and moisturizing bacte riostatic for detergent or nondetergent cosmetics and toiletries containing 1.2-octanediol (referring to publication No of Published Japanese patent application) are disclosed. In using 1.2-alkanediol Such as 1.2-octanediol solely for the antiseptic disinfectant, 1,2-alkanediol has to be compounded in a large amount if the nonionic Surfactant exists in the compound. Further, as the 1,2-alkanediol has a peculiar odor of its material, it causes the problem of the odor when compounded in cosmetics and toiletries, etc. Still further, for the technical means as to the antiseptic disinfectant using 1.2-alkanediol, a composition for external use compounded with 1.2-pentanediol and 2-phenoxyethanol (referring to publication No of Published Japanese patent application) and an antiseptic disinfectant containing 1,2-alkanediol and paraben (referring to publication No of Published Japanese patent application) are dis closed. The aims of those are, however, to improve the effect of antiseptic by the combination use of antiseptic and 1.2- alkanediol Such as 1.2-pentanediol, but not to completely remove the antiseptic itself. Meanwhile, fragrance compositions are generally com pounded in the cosmetics and toiletries and food etc. to give rich Smell to the products. It is known since a long time ago that the fragrance compositions theirselves have antibacterial action, and for example, the fragrance composition Such as cresyl acetate, methyl eugenol, heliotropin and ethyl salicy late is disclosed as anticaries that has antibacterial action against mutans (referring to Published Japanese patent pub lication No ). However, there has been a problem that the antibacterial action only by the fragrance composi tion does not give enough antiseptic disinfectant effect for cosmetics, toiletries and so on. In view of the above-mentioned circumstances, the inven tors, after studying very hard, found that combination use of 1.2-alkanediol and a specific fragrance composition enhances the antibacterial activity which 1,2-alkanediol originally has against many different kinds of strains to mask the odor of 1,2-alkanediol material, thereby the present invention has been completed. DISCLOSURE OF THE INVENTION A first embodiment of the invention relates to an antiseptic disinfectant including 1,2-alkanediol with 5-10 carbons, and one or more materials selected from a group of thymol. eugenol, citronellal, terpinyl acetate, citronellol and B-pinene. A second embodiment of the invention relates to an anti septic disinfectant described in the first embodiment, wherein 1.2-alkanediol is 1.2-hexanediol and/or 1.2-octanediol. A third embodiment of the invention relates to an antiseptic disinfectant described in the first embodiment, wherein the 1.2-alkanediol is 1.2-octanediol. A fourth embodiment of the invention relates to cosmetics and toiletries including the antiseptic disinfectant described in the first embodiment. A fifth embodiment of the invention relates to cosmetics and toiletries including the antiseptic disinfectant described in the second embodiment. A sixth embodiment of the invention relates to cosmetics and toiletries including the antiseptic disinfectant described in the third embodiment. A seventh embodiment of the invention relates to medicine including the antiseptic disinfectant described in the first embodiment. An eighth embodiment of the invention relates to medicine including the antiseptic disinfectant described in the second embodiment. A ninth embodiment of the invention relates to medicine including the antiseptic disinfectant described in the third embodiment. A tenth embodiment of the invention relates to food includ ing the antiseptic disinfectant described in the first embodi ment. An eleventh embodiment of the invention relates to food including the antiseptic disinfectant described in the second embodiment. A twelfth embodiment of the invention relates to food including the antiseptic disinfectant described in the third embodiment. BRIEF DESCRIPTION OF THE DRAWINGS FIG. 1 is a diagram showing an example of a method to determine the effect of compounding two antibacterial mate rials by means of dual minimum inhibitory concentration. FIG. 2 is dual minimum inhibitory concentration diagrams as to thymol. FIG. 3 is dual minimum inhibitory concentration diagrams as to eugenol. FIG. 4 is dual minimum inhibitory concentration diagrams as to citronellal.
11 3 FIG. 5 is dual minimum inhibitory concentration diagrams as to terpinyl acetate. FIG. 6 is dual minimum inhibitory concentration diagrams as to citronellol. FIG. 7 is dual minimum inhibitory concentration diagrams as to B-pinene. FIG. 8 is dual minimum inhibitory concentration diagrams as to isobornyl acetate. FIG.9 is dual minimum inhibitory concentration diagrams as to guajac acetate. BEST MODE FOR CARRYING OUT THE INVENTION Hereinafter, the antiseptic disinfectant of the present inven tion is disclosed in detail. The antiseptic disinfectant of the present invention contains 1,2-alkanediol with 5-10 carbons, and one or more materials selected from a group of thymol. eugenol, citronellal, terpinyl acetate, citronellol and B-pinene. The first component of the antiseptic disinfectant of the present invention is 1,2-alkanediol with 5-10 carbons as indi cated in the following chemical formula 1, and more specifi cally, it can be 1.2-pentanediol. 1.2-hexanediol. 1.2-hep tanediol. 1.2-octanediol. 1.2-nonanediol, or 1.2-decanediol. CH-CH-(CH)n-CH OH OH (Formula 1) ( n in the formula represents integer 2-7) In the present invention, one of the above-mentioned 1,2- alkanediols can be used solely, or two or more of those can be compounded. 1,2-alkanediol itself has a fine antibacterial activity, and takes effect of enhancing the antibacterial activity of the antiseptic disinfectant. Especially, in the present invention, it is preferable to use 1.2-hexanediol or 1,2-octanediol out of 1,2-alkanediols with 5-10 carbons as they have superior anti bacterial activity against Eumycetes Such as bacteria, yeast, Fungi and so on. It would be more preferable to use 1.2- octanediol. The second component of the antiseptic disinfectant of the present invention is one or more materials selected from a group of thymol, eugenol, citronellal, terpinyl acetate, cit ronellol and B-pinene. The second component is the fragrance composition which not only masks the peculiar odor of 1.2-alkanediol but also enhances the antibacterial activity. Thymol is achromatic crystal or crystalline which has a molecular formula of CoHO, aromatic odor like thyme oil and acrid taste. Thymol also has a fine antiseptic and bacte ricidal activity. Thymol can be obtained by extracting from essential oil such as ajowan oil and the like, or by the reaction of m-cresol at the existence of isopropyl chloride and alu minium chloride under cooled condition. Eugenol is light yellow-colored liquid which has a molecu lar formula of CoH2O and the aromatic odor like clove oil. Eugenol is included in essential oil such as clove oil, cassia oil, pimento oil, bay oil, camphor oil and so on. Eugenol can be obtained by extracting phenol from the essential oil by means of dilute alkaline solution and neutralizing it by means of mineral acid or carbons dioxide. Citronellal is achromatic or light yellow-colored liquid which has a molecular formula of CoHO and the aromatic odor like lemon. It is included in citronella oil, melissa oil and so on. It can be obtained by the process of fractional distilla tion of the essential oil, processing with acid sodium sulfite to prepare the crystalline addition compound, cleaning with flux to remove adulterants, decomposing it with alkali carbonsate, and refining after steam distillation and vacuum distillation. Terpinyl acetate is achromatic liquid which has a molecular formula of C2H2O and the strong aromatic odor like ber gamot and lavender. It can be obtained by heating pinene for many hours with excessive amount of acetic acid, or by boil ing terpineol diluted by dimethylbenzene using a reflux con denser of acetic anhydride and sodium acetate. Citronellol is achromatic liquid which has a molecular formula of CHO and the aromatic odor like roses. It is included in citronella oil, Geranium oil, rose oil and so on. It can be obtained by reduction of citronellal. B-pinene is achromatic liquid which has a molecular for mula of CoH. It is important as a starting raw material for synthetic perfumes, and is included interpentine oil. In the present invention, one material from a group of thymol, eugenol, citronellal, terpinyl acetate, citronellol and B-pinene can be used solely, or two or more of those can be compounded. In the antiseptic disinfectant of the present invention, the content of both of the first component 1,2-alkanediol with 5-10 carbons and the second component fragrance composi tions are not limited, but it would be preferred that they are compounded to satisfy the weight ratio to be between 0.5:1 and 100:1, more preferably between 1:1 and 10:1. It is not preferable to compound 1,2-alkanediol over 100 times as much as fragrance components by weight because the effect of enhancing antibacterial activity cannot be expected in the condition, while it is not preferable to compound 1,2-al kanediol under 0.5 times as much by weight because the aromatic odor increases too much in the condition. The antiseptic disinfectant includes 1,2-alkanediol as the first component, and one or more materials selected from a group of thymol, eugenol, citronellal, terpinyl acetate, cit ronellol and 3-pinene as the second component, it masks the raw material odor of 1,2-alkanediol. At the same time, fine antiseptic disinfectant activity against bacterias, yeast, fungi and so on can be shown because of the multiplier effect by the first and second components as indicated in the test men tioned below. Consequently, it is not necessary to compound the conventional antiseptic disinfectant such as paraben, ben Zoic acid and salicylic acid, thereby the high level of safety is secured. The above-mentioned antiseptic disinfectant of the present invention can be used for the composition of cosmetics and toiletries, medicine, or food. Specifically, it can be preferably used for skin cosmetics and toiletries Such as facial cleansing, skin lotion, emulsion, cream, foundation, mascara, nail enamel and rouge, for hair cosmetics and toiletries Such as shampoo, hair treatment, hair growth stimulant, hair cream, hair lotion, hair foam, perma nent wave agent, for medicinal cosmetics (quasi drugs) for a definite purpose Such as chloasma or ephelides, for medicine Such as curative medicine for acne, collutorium or troches, and further for food such as chewing gums, candies or bev erages. In preparing cosmetics and toiletries, medicine or food with the antiseptic disinfectant of the present invention, the compositions which are usually contained in the cosmetics, toiletries, medicine or food can optionally be compounded within the amount that does not spoil the effect of the present invention. For example, fat, cere, higher fatty acid, lower, alcohol, higher alcohol, Sterol, fatty acid ester, humectant
12 5 agent, Surfactant, high molecular compound, inorganic pig ment, coloring matter, fragrance composition, antioxidant, ultraviolet absorber, vitamin, astringent, skin whitening agent, extract from animals and plants, sequestering agent, purified water and so on would be enumerated for cosmetics, toiletries or medicine (including quasi drugs). As to food, oil of animals and plants, polysaccharide, Sweetener, food color, gum base and so on can be enumerated for example. In preparing cosmetics, toiletries, medicine or food, the content of the antiseptic disinfectant is not limited, but the composition contains percent by weight of the anti septic disinfectant, and, more preferably, contains per cent by weight of it. If more than 20 percent by weight of the antiseptic disinfectant is contained, additional effect would not be expected. While, if contained under 0.01 percent by weight, it would not be preferable because the enough anti bacterial effect cannot be obtained. EMBODIMENT Embodiment 1 Candida albicans IFO1594 (mycotic stomatitis), Staphy lococcus aureus IFO13276 (Staphylococcus aureus), and Pseudomonas aeruginosa IFO13275 (Pseudomonas) are used as sample bacterias. (Preparation of Inoculating Bacteria) For preparing bacteria, as to Staphylococcus aureus and Pseudomonas, they are incubated at 35 C. in the agar medium, and further incubated at 35 C. after transferred into the bouillon medium. The inoculating bacteria is prepared by diluting the obtained culture solution to about 10 inoculating cell /ml. As to yeast (Candida albicans), the inoculating bacteria is prepared by incubating the yeast in the same way as Staphy lococcus aureus and Pseudomonas at 30 C. and diluting the yeast to about 107 cell/ml. (Preparation of Diluting Series of the Test Material) With a dilution solvent of ethyl cellosolve of 20 w/w %, 1.2-octanediol solution of 5, 4, 3, 2.5, 2.25, 2, 1.75, 1.5, 1.25 and 1 w/v '% are prepared. While, as to thymol and the 50:50 by weight mixture of 1.2-octanediol and thymol, the dilution series are prepared by doubling diluting the 20w/v '% solution. (Measurement of Minimum Inhibitory Concentration (MIC)) 1 ml of each of the above-mentioned dilution series includ ing the test material and 9 ml of the agar medium are intro duced to each Schales, and the above-mentioned inoculating bacteria was applied on each of the Schales at the length of 1 C. Staphylococcus aureus and Pseudomonas were incubated at 35 C., and it was judged whether or not the bacteria had grown two days later. The yeast was incubated at 25 C., and it was judged whether or not the bacteria had grown three days later. The minimum concentration in which the bacteria did not grow is MIC. Still more, antibacterial activity can be evaluated by the MIC. When the concentration of the test material is low, it does not effect the microbes. As the concentration gets higher, growth inhibition occurs. Further, as the concentration gets higher, the growth inhibition progresses, eventually to stop growth. The concentration at the point is shown as MIC. Accordingly, when the concentration goes above MIC, microbes die (Evaluation of Dual Minimum Inhibitory Concentration) Each MIC of the obtained 1,2-octanediol, thymol, and the 50:50 mixture by weight of 1,2-octanediol and thymol corre sponding the compounding amount of 1.2-octanediol and thymol is plotted to make out the dual minimum inhibitory concentration. Action and effect in case that two kinds of antibacterial materials are compounded can be judged by the dual minimum inhibitory concentration. More specifically, the actions caused by compounding two antibacterial mate rials are roughly classified into synergistic action, additive action and counteraction. Synergistic action is the action in that two kinds of agent act synergistically, to enhance the antibacterial activity that the agents originally have. The addi tive action is the action in that the antibacterial activities of the agents are put together. The counteraction is the action in that one agent cancels the antibacterial activity of the other agent. The method using dual minimum inhibitory concentration, as shown in FIG. 1 for example, is the method to measure MICs as to material A and Material Bat different proportions of them and to judge the result in view of the graph. In this method, a line is drawn to connect MIC (point A) in using only material Ato MIC (point B) in using only material B. When the MIC (point C) in using both materials is inside the line, it is judged to be Synergistic action that antibacterial activity was strengthened by the combined use. When the MIC (point D) is on the line, it can be judged to be the additive action. When the MIC (point E) is in the outside of the line, it is judged to be the counteraction that cancels antibacterial activity of one or both of the materials to decrease the anti bacterial activity. The dual minimum inhibitory concentration diagram as to the thymol is shown in FIG. 2. (Evaluation of the Antibacterial Effect) The antibacterial effect in the case of mixing 1,2-oc tanediol and thymol was evaluated by the following evalua tion criterion from dual minimum inhibitory concentration diagram in FIG. 2. The result is described in the table 1. <Evaluation Criterion O: There is synergistic action in the antibacterial effect. A: There is additive action in the antibacterial effect. x: There is counteraction in the antibacterial effect. Embodiment 2 Manipulating each of eugenol, isobornyl acetate and guaiac acetate in the same way as in Embodiment 1, each dual minimum inhibitory concentration of FIG.3, FIG. 8 and FIG. 9 was obtained. From the obtained result, the antibacterial effect was evaluated by the above-mentioned evaluation cri terion. The result is shown in the table 1. TABLE 1 fragrance component C. albicans S. aureus Paeruginosa Embodiment 1 Thymol O O O Embodiment 2 Eugenol O O O Comparative isobornyl X O X example 1 acetate Comparative guaiac X O X example 2 acetate Embodiment 3-4 Manipulating each of citronellal and terpinyl acetate for fragrance component the same way as in Embodiment 1 using
13 7 Candida albicans IFO1594 and Staphylococcus aureus IFO13276, each dual minimum inhibitory concentration of FIGS. 4-5 was obtained. From the obtained result, the anti bacterial effect was evaluated by the above-mentioned evalu ation criterion. The result is shown in the table 2. TABLE 2 fragrance component C. albicans S. airetts Embodiment 3 citronellal O O Embodiment 4 terpinyl acetate O O Comparative example 1 isobornyl acetate X O Comparative example 2 guaiac acetate X O Embodiment 5-6 Manipulating each of citronellol and B-pinene for fra grance component in the same way as in Embodiment 1 using Escherichia coli IFO3972 instead of Pseudomonas aerugi nosa IFO13275, each dual minimum inhibitory concentration of FIGS. 6-7 was obtained. Each dual minimum inhibitory concentration of isobornyl acetate and guaiac acetate against Escherichia coli was obtained in the same way. From the obtained result, the antibacterial effect was evaluated by the above-mentioned evaluation criterion. The result is shown in the table 3. TABLE 3 fragrance component C. albicans S. aurents E. coi Embodiment 1 citronellol O O O Embodiment 2 B-pinene O O A Comparative isobornyl acetate X O X example 1 Comparative guaiac acetate X O X example 2 From the result shown in the Table 1-3, it can be known that it enhances the antibacterial activity which 1,2-alkanediol originally has against a broad range of strains to use 1.2- alkanediol in combination with a certain fragrance compo nent Such as thymol, eugenol, citronellal, terpinyl acetate, citronellol and B-pinene. On the other hand, it can be known that using 1,2-al kanediol in combination with other fragrance component Such as isobornyl acetate or guaiac acetate does not enhance the antibacterial activity against a broad range of strains. Examples of the compound of antiseptic disinfectant, and cosmetics and toiletries, medicine or food containing the same according to the present invention are described as follows. <Formulation example 1: Humidity retention cream decaglyceryl monolaurate 1.O POE (15) glyceryl monostearate 1.O Soybean phosphatide for hydrogenation 1.O Stearic acid 4.0 cetanol 2.0 behenyl alcohol 2.0 paraffin 3.0 Squalane 12.O jojoba oil 4.0 methyl polysiloxane O continued <Formulation example 1: Humidity retention cream 1,3-butylene glycol 3.0 L-arginine O.1 Xanthan gum O.OO1 1,2-octanediol O.25 eugenol O.1 purified water proper quantity total <Formulation example 2: hydrophilic ointment> ascorbic acid O.S polyoxyethylene cetyl ether 2.0 Soybean phosphatide for hydrogenation 1.O Stearic acid 4.0 glyceric monostearate liquid paraffin 1O.O 1O.O Vaseline 4.0 cetanol S.O propylene glycol S.O hexanediol O.S 100 percent by weight citronellal O.2 purified water proper quantity total <Formulation example 3: beverage-> dextrose syrup 33.0 grapefruit juice 64.O 1.2-pentanedio O.S citronellol O.O1 fragrance O.S acidulant proper quantity total 100 percent by weight 100 percent by weight Effect of the Invention As disclosed above, the antiseptic disinfectant of the present invention enhances the antibacterial activity that 1.2- alkanediol originally have againstabroad range of the strains. Accordingly, it is not necessary to compound the conven tional antiseptic disinfectant, and high degree of safety can be obtained. Further, when 1.2-hexanediol or 1,2-octanediol is used for 1.2-alkanediol, the antiseptic disinfectant with higher anti bacterial activity can be obtained. The cosmetics and toiletries, medicine or food of the present invention do not need to contain the conventional antiseptic disinfectant Such as Salicylic acid, benzoic acid or paraben, and the antiseptic disinfectant of the present inven tion has a excellent antibacterial activity. Accordingly, the compounding rate of the antiseptic disinfectant itself can be low, and the safety is extremely high. INDUSTRIAL APPLICABILITY The antiseptic disinfectant of the present invention enhances the antibacterial activity that 1,2-alkanediol origi nally have against a broad range of the strains, and has high
14 9 degree of safety. Therefore, it can be suitably used as the antiseptic disinfectant for cosmetics and toiletries, medicine or food. What is claimed is: 1. An antiseptic disinfectant, containing 1.2-octanediol, and one or more materials selected from the group consisting of citronellal, terpinyl acetate, citronellol and B-pinene; wherein the weight ratio of said 1.2-octanediol and said one or more materials is between 0.5:1 and 10:1. 2. Cosmetics and toiletries comprising: a cosmetically effective agent, 1.2-octanediol, and 15 one or more materials selected from the group consisting of citronellal, terpinyl acetate, citronellol and B-pinene; wherein the weight ratio of said 1.2-octanediol and said one or more materials is between 0.5:1 and 10:1, and - 2O wherein said cosmetics and toiletries are Suitable for use on humans or animals Medicine comprising: a medicinally effective agent, 1.2-octanediol, and one or more materials selected from the group consisting of eugenol, citronellal, terpinyl acetate, citronellol and B-pinene; wherein the weight ratio of said 1.2-octanediol and said one or more materials is between 0.5:1 and 10:1, and wherein said medicine is medicinally effective on humans or animals. 4. Food, comprising: a nutritionally effective edible substance, 1.2-octanediol, and one or more materials selected from the group consisting of eugenol, citronellal, terpinyl acetate, citronellol and B-pinene; wherein the weight ratio of said 1.2-octanediol and said one or more materials is between 0.5:1 and 10:1, and wherein said food is edible and non-toxic to humans or animals.
(12) United States Patent (10) Patent No.: US 6,752,627 B2
 USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
(12) United States Patent (10) Patent No.: US 6,841,523 B1
 USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150343112A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0343112 A1 Dyar et al. (43) Pub. Date: (54) LIQUID BANDAGE Publication Classification (71) Applicant: PROBLEM
(19) United States US 20150343112A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0343112 A1 Dyar et al. (43) Pub. Date: (54) LIQUID BANDAGE Publication Classification (71) Applicant: PROBLEM
II. Moisturization Improves skin moisture content General Recognized as Safe (GRAS) ingredients by FDA (21 CFR 184) No animal testing, non GMO.
 Lonza Ltd Muenchensteinerstrasse 38 CH-4002 Basel, Switzerland Tel +41 61 316 81 11 Fax +41 61 316 91 11 Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries,
Lonza Ltd Muenchensteinerstrasse 38 CH-4002 Basel, Switzerland Tel +41 61 316 81 11 Fax +41 61 316 91 11 Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries,
Product Information Gluconolactone and Sodium Benzoate (GSB)
 The Soap Kitchen Unit 8 Caddsdown Industrial Park, Clovelly Road, Bideford, Devon EX39 3DX Tel: 01237 420872 (+44 (0)1237 420872) Email: info@thesoapkitchen.co.uk Product Information Gluconolactone and
The Soap Kitchen Unit 8 Caddsdown Industrial Park, Clovelly Road, Bideford, Devon EX39 3DX Tel: 01237 420872 (+44 (0)1237 420872) Email: info@thesoapkitchen.co.uk Product Information Gluconolactone and
III. United States Patent 19 Jordan 5,389,129. Feb. 14, ). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee:
 United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
PRODEW P-DS-12. a Nature-inspired Moisturizer with Biostabilizing Properties
 PRODEW P-DS-12 a Nature-inspired Moisturizer with Biostabilizing Properties 1 Contents Demand for Preservatives Technical challenge Market trend PRODEW P-DS-12 Characteristics Moisturizing property Biostabilizing
PRODEW P-DS-12 a Nature-inspired Moisturizer with Biostabilizing Properties 1 Contents Demand for Preservatives Technical challenge Market trend PRODEW P-DS-12 Characteristics Moisturizing property Biostabilizing
Demystifying Skin Care for Massage Therapists Chapter 5
 1 Demystifying Skin Care for Massage Therapists Chapter 5 Created by Nina Howard, Founder and Master Trainer Adapted and Edited by Kathryn Myers, CEO Bellanina Insitute BELLANINA INSTITUTE for Skin and
1 Demystifying Skin Care for Massage Therapists Chapter 5 Created by Nina Howard, Founder and Master Trainer Adapted and Edited by Kathryn Myers, CEO Bellanina Insitute BELLANINA INSTITUTE for Skin and
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
(12) United States Patent
 US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
Innovative Preservative Solutions. Dr. Matthias Kunze LANXESS Distribution GmbH Barcelona, 16 April 2015
 Innovative Preservative Solutions Dr. Matthias Kunze LANXESS Distribution GmbH Barcelona, 16 April 2015 Innovative Preservative Solutions Bild/Grafik Größe: Topics Status of cosmetic preservation Innovative
Innovative Preservative Solutions Dr. Matthias Kunze LANXESS Distribution GmbH Barcelona, 16 April 2015 Innovative Preservative Solutions Bild/Grafik Größe: Topics Status of cosmetic preservation Innovative
PCA derivatives. Our performant range of humectants
 PCA derivatives Our performant range of humectants enews August 2015 What is PCA? Pyrrolidone carboxylic Acid (PCA) is one of the component of the Natural Moisturizing Factor (NMF) NMF is the natural moisturizing
PCA derivatives Our performant range of humectants enews August 2015 What is PCA? Pyrrolidone carboxylic Acid (PCA) is one of the component of the Natural Moisturizing Factor (NMF) NMF is the natural moisturizing
B & B Co., LTD Do our Best, Be the Best!
 B & B Co., LTD Do our Best, Be the Best! Specialty Chemicals Construction Chemicals Cosmetic Chemicals Specialized in the Construction & Cosmetic Chemicals www.bnbchem.com Greeting B&B Co.,LTD are established
B & B Co., LTD Do our Best, Be the Best! Specialty Chemicals Construction Chemicals Cosmetic Chemicals Specialized in the Construction & Cosmetic Chemicals www.bnbchem.com Greeting B&B Co.,LTD are established
Skin knows the difference
 Skin knows the difference Cleanse Protect Treat Moisturize SECURA is a proven four-step preventive skin care system. SECURA products are formulated with high-quality ingredients that define the standard
Skin knows the difference Cleanse Protect Treat Moisturize SECURA is a proven four-step preventive skin care system. SECURA products are formulated with high-quality ingredients that define the standard
1,3-Dimethylol-5,5-dimethylhydantoin 3-Iodo-2-propynyl butyl carbamate
 Product Information Glydant Plus Liquid The liquid high performance preservative system. Synergistic, broad spectrum mode of action: no need for auxiliary preservatives. ighly cost-effective use level.
Product Information Glydant Plus Liquid The liquid high performance preservative system. Synergistic, broad spectrum mode of action: no need for auxiliary preservatives. ighly cost-effective use level.
(12) United States Patent (10) Patent No.: US 9, B2
 USOO9566228B2 (12) United States Patent (10) Patent No.: US 9,566.228 B2 Jeong (45) Date of Patent: Feb. 14, 2017 (54) BUBBLE TYPE WATERLESS SHAMPOO (2013.01); A61K 8/442 (2013.01); A61K 8/64 COMPOSITION
USOO9566228B2 (12) United States Patent (10) Patent No.: US 9,566.228 B2 Jeong (45) Date of Patent: Feb. 14, 2017 (54) BUBBLE TYPE WATERLESS SHAMPOO (2013.01); A61K 8/442 (2013.01); A61K 8/64 COMPOSITION
Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries.
 Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. I. Preservation Naturally-derived product Broad spectrum protection Globally accepted II. Moisturization
Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. I. Preservation Naturally-derived product Broad spectrum protection Globally accepted II. Moisturization
Natural Preservatives
 Cosmetic Natural Preservatives 1. MSE-G 2. BHC-C MSE-G Natural preservatives that could apply to all cosmetic products With wide anti-microbiological activity Viscosity is not affected No reaction with
Cosmetic Natural Preservatives 1. MSE-G 2. BHC-C MSE-G Natural preservatives that could apply to all cosmetic products With wide anti-microbiological activity Viscosity is not affected No reaction with
(12) United States Patent
 US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
United States Patent (19) Katz
 United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
OxyBAC. Antimicrobial rich-cream foam hand wash. Kills % of many common germs
 OxyBAC Antimicrobial rich-cream foam hand wash Kills 99.999% of many common germs Leading the fight against occupational skin disorders All Occupational Skin Care Needs in One Range Combining innovation,
OxyBAC Antimicrobial rich-cream foam hand wash Kills 99.999% of many common germs Leading the fight against occupational skin disorders All Occupational Skin Care Needs in One Range Combining innovation,
(12) United States Patent (10) Patent No.: US 9,468,596 B2
 USOO946896B2 (12) United States Patent () Patent No.: Eizen et al. () Date of Patent: Oct. 18, 2016 (4) MULTI USE COSMETIC FORMULA (2013.01); A61K 8/73 (2013.01); A61K 8/737 (2013.01); A61O 1/00 (2013.01);
USOO946896B2 (12) United States Patent () Patent No.: Eizen et al. () Date of Patent: Oct. 18, 2016 (4) MULTI USE COSMETIC FORMULA (2013.01); A61K 8/73 (2013.01); A61K 8/737 (2013.01); A61O 1/00 (2013.01);
Represented by: Integrity Ingredients Corporation
 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 AMPHOTERIC
Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 AMPHOTERIC
All Natural Ingredients for DIY Skincare
 All Natural Ingredients for DIY Skincare In the Fridge: Eggs Yogurt Lemon Protein- For tissue repair and growth. Promotes strong, wrinkle-free skin and shiny hair. Potassium-Hydrates and moisturizes. Preserves
All Natural Ingredients for DIY Skincare In the Fridge: Eggs Yogurt Lemon Protein- For tissue repair and growth. Promotes strong, wrinkle-free skin and shiny hair. Potassium-Hydrates and moisturizes. Preserves
AMINAT -G PRESERVATIVE AND ACTIVE ANTIMICROBIAL FOR COSMETICS COMPOSITION
 AMINAT -G PRESERVATIVE AND ACTIVE ANTIMICRBIAL FR CSMETICS INCI name: Glycerin and Ethyl Lauroyl Arginate HCl CMPSITIN AMINAT -G is based on a 20% solution of LAE (Ethyl lauroyl arginate monohydrochloride,
AMINAT -G PRESERVATIVE AND ACTIVE ANTIMICRBIAL FR CSMETICS INCI name: Glycerin and Ethyl Lauroyl Arginate HCl CMPSITIN AMINAT -G is based on a 20% solution of LAE (Ethyl lauroyl arginate monohydrochloride,
(12) United States Patent (10) Patent No.: US 6,413,305 B1
 USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
(12) United States Patent (10) Patent No.: US 8,770,209 B2
 US008770209B2 (12) United States Patent (10) Patent No.: Kim et al. (45) Date of Patent: Jul. 8, 2014 (54) COLOR HIGHILIGHTING COSMETICS (52) U.S. Cl. CONTAINER INCLUDING ADETACHABLE USPC... 132/297: 132/318;
US008770209B2 (12) United States Patent (10) Patent No.: Kim et al. (45) Date of Patent: Jul. 8, 2014 (54) COLOR HIGHILIGHTING COSMETICS (52) U.S. Cl. CONTAINER INCLUDING ADETACHABLE USPC... 132/297: 132/318;
(12) United States Patent
 (12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
(12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
(12) United States Patent
 USOO9639 120B2 (12) United States Patent W (10) Patent No.: (45) Date of Patent: May 2, 2017 (54) HEAT DISSIPATIONSTRUCTURE OF WEARABLE ELECTRONIC DEVICE (71) Applicant: ASIA VITAL COMPONENTS CO., LTD.,
USOO9639 120B2 (12) United States Patent W (10) Patent No.: (45) Date of Patent: May 2, 2017 (54) HEAT DISSIPATIONSTRUCTURE OF WEARABLE ELECTRONIC DEVICE (71) Applicant: ASIA VITAL COMPONENTS CO., LTD.,
(12) United States Patent (10) Patent No.: US 6,308,717 B1
 USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
(12) (10) Patent No.: US 6,971,424 B1. Angevine (45) Date of Patent: Dec. 6, (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi...
 United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
(12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001
 US006257248B1 (12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001 (54) BOTH HAND HAIR CUTTING METHOD 5,991,918 * 11/1999 Choate..... 2/21 6,079,107 * 6/2000
US006257248B1 (12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001 (54) BOTH HAND HAIR CUTTING METHOD 5,991,918 * 11/1999 Choate..... 2/21 6,079,107 * 6/2000
Transforming The Face Of Preservation: Peptide Technology In The Personal Care Industry Antimicrobials A Natural Approach to Product Preservation
 In The Personal Care Industry Antimicrobials A Natural Approach to Product Preservation Anna Crovetto - Technical Marketing - Email: a.crovetto@activeconcepts.it Contents 1. Preservation: Chemist vs. Consumer
In The Personal Care Industry Antimicrobials A Natural Approach to Product Preservation Anna Crovetto - Technical Marketing - Email: a.crovetto@activeconcepts.it Contents 1. Preservation: Chemist vs. Consumer
SEBUSPOT. distributor. product profiles β-active product profiles
 β-active SEBUSPOT DESCRIPTION: Environ s β-active Sebuspot is a mild, effective gel with Australian Tea Tree Oil know for its antibacterial properties. It is designed for use on individual blemishes and
β-active SEBUSPOT DESCRIPTION: Environ s β-active Sebuspot is a mild, effective gel with Australian Tea Tree Oil know for its antibacterial properties. It is designed for use on individual blemishes and
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
(19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
Europaisches Patentamt European Patent Office. Publication number: Office europeen des brevets EUROPEAN PATENT APPLICATION
 J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
Ref. 11. (12) Patent Application Publication (10) Pub. No.: US 2012/ A1. (19) United States. Polstein et al. (43) Pub. Date: Jun.
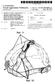 (19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
Performance is in our nature.
 Performance is in our nature. Propanediol for Skin Care propanediol is a natural, skin-friendly, propanediol is ideally suited for many different skin preservative-boosting alternative to petroleum-based
Performance is in our nature. Propanediol for Skin Care propanediol is a natural, skin-friendly, propanediol is ideally suited for many different skin preservative-boosting alternative to petroleum-based
MIGLYOL 840. Excellent light emollient. Alternative to IPM. INCI: Propylene Glycol Dicaprylate/Dicaprate. Excellent light and dry emollient
 Excellent light emollient Excellent light and dry emollient Alternative to IPM 1. Description: MIGLYOL 840 is a propylene glycol diester of saturated plant fatty acids with chain lengths of C 8 and C 10.
Excellent light emollient Excellent light and dry emollient Alternative to IPM 1. Description: MIGLYOL 840 is a propylene glycol diester of saturated plant fatty acids with chain lengths of C 8 and C 10.
(12) United States Patent (10) Patent No.: US 7434,929 B2
 US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
(19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
Zemea Propanediol : Optimizing Formulations Using a Natural Solvent and Humectant. Skincare Ingredients 2013 June 12, 2013
 Zemea Propanediol : Optimizing Formulations Using a Natural Solvent and Humectant Skincare Ingredients 2013 June 12, 2013 Denis Burlaud Account Manager, Europe Bob Miller, Technical Consultant DuPont Tate
Zemea Propanediol : Optimizing Formulations Using a Natural Solvent and Humectant Skincare Ingredients 2013 June 12, 2013 Denis Burlaud Account Manager, Europe Bob Miller, Technical Consultant DuPont Tate
Tomura, Kazuyo Osaka-shi, Osaka-fu (JP)
 * OJII Eur Pean Patent Office mi mi ii mi 111 ii mi 111 iii Office eurodeen des brevets (11) E P 0 7 1 6 8 4 6 A 1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51 ) nt. CI.6: A61 K 7/1 3
* OJII Eur Pean Patent Office mi mi ii mi 111 ii mi 111 iii Office eurodeen des brevets (11) E P 0 7 1 6 8 4 6 A 1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51 ) nt. CI.6: A61 K 7/1 3
United States Patent (19) Hawkins et al.
 United States Patent (19) Hawkins et al. 11 Patent Number: ) Date of Patent: 4,6,043 Apr. 7, 1987 54) PEROXIDE-CONTAINING CONDITIONING SHAMPOO 75) Inventors: Geoffrey R. Hawkins, Cheshire; Oksana A. Kowcz,
United States Patent (19) Hawkins et al. 11 Patent Number: ) Date of Patent: 4,6,043 Apr. 7, 1987 54) PEROXIDE-CONTAINING CONDITIONING SHAMPOO 75) Inventors: Geoffrey R. Hawkins, Cheshire; Oksana A. Kowcz,
Irwin Palefsky Cosmetech Laboratories Inc.
 Irwin Palefsky Cosmetech Laboratories Inc. Irwin@cosmetech.com www.cosmetech.com The prevention or retardation of product deterioration caused by microorganisms from the time of production until the product
Irwin Palefsky Cosmetech Laboratories Inc. Irwin@cosmetech.com www.cosmetech.com The prevention or retardation of product deterioration caused by microorganisms from the time of production until the product
American Cleaning Institute Development of Exposure Assessments Glossary of Functional Classes
 American Cleaning Institute Development of Exposure Assessments Glossary of Functional Classes Abrasive: Abrasive ingredients are materials that are used to polish, buff, or scour away soils such as dirt
American Cleaning Institute Development of Exposure Assessments Glossary of Functional Classes Abrasive: Abrasive ingredients are materials that are used to polish, buff, or scour away soils such as dirt
(12) United States Patent (10) Patent No.: US 7.427,133 B2
 USOO7427133B2 (12) United States Patent (10) Patent No.: US 7.427,133 B2 Carter (45) Date of Patent: Sep. 23, 2008 (54) EARPIECE-LESS EYEGLASS FRAME 5.313,671 A * 5/1994 Flory... 2,428 HAVING AREMOVABLE
USOO7427133B2 (12) United States Patent (10) Patent No.: US 7.427,133 B2 Carter (45) Date of Patent: Sep. 23, 2008 (54) EARPIECE-LESS EYEGLASS FRAME 5.313,671 A * 5/1994 Flory... 2,428 HAVING AREMOVABLE
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
Preservative Effects of Zemea Propanediol
 Preservative Effects of Zemea Propanediol In-Cosmetics 2012 Barcelona Thursday, April 19 Denis Burlaud, EMEA Sales Manager DuPont Tate & Lyle Bio Products Joint venture formed in 2004 between DuPont and
Preservative Effects of Zemea Propanediol In-Cosmetics 2012 Barcelona Thursday, April 19 Denis Burlaud, EMEA Sales Manager DuPont Tate & Lyle Bio Products Joint venture formed in 2004 between DuPont and
(12) United States Patent (10) Patent No.: US 7,585,200 B1
 US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
Hand Care Product Systems. Your hands have been in good hands for over 60 years...
 Hand Care Product Systems Your hands have been in good hands for over 60 years... Our World Class Hand Care Solutions... NCL Hand Care Product Systems Healthcare professionals and community leaders universally
Hand Care Product Systems Your hands have been in good hands for over 60 years... Our World Class Hand Care Solutions... NCL Hand Care Product Systems Healthcare professionals and community leaders universally
State of the art ingredients fast friendly service
 Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. Description Geogard Ultra is accepted by ECOCERT as a preservative in certified organic cosmetics. As
Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. Description Geogard Ultra is accepted by ECOCERT as a preservative in certified organic cosmetics. As
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States US 20130244977A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0244977 A1 Lee et al. (43) Pub. Date: (54) COSMETIC BIO-CELLULOSE MASK PACK (30) Foreign Application Priority
(19) United States US 20130244977A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0244977 A1 Lee et al. (43) Pub. Date: (54) COSMETIC BIO-CELLULOSE MASK PACK (30) Foreign Application Priority
ACB Botanical Sugar Complex
 ACB Botanical Sugar Complex Moisturization + Antioxidant + Hair Nourishing Tomorrow s Vision Today! ACB Botanical Sugar Complex Technical Information: Product Code: 20039 INCI Name: Tapioca Starch and
ACB Botanical Sugar Complex Moisturization + Antioxidant + Hair Nourishing Tomorrow s Vision Today! ACB Botanical Sugar Complex Technical Information: Product Code: 20039 INCI Name: Tapioca Starch and
Sugar based, very efficient emulsifier for PEG-free O/W lotions and creams
 Technical Information TEGO Care CG 90 Sugar based, very efficient emulsifier for PEG-free O/W lotions and creams Intended use O/W emulsifier Benefits at a glance Emulsifier for "natural" O/W emulsions
Technical Information TEGO Care CG 90 Sugar based, very efficient emulsifier for PEG-free O/W lotions and creams Intended use O/W emulsifier Benefits at a glance Emulsifier for "natural" O/W emulsions
Ecolab Total Hand Hygiene System Clean without a doubt.
 Ecolab Total Hand Hygiene System Clean without a doubt. WHY AREN T YOUR EMPLOYEES GETTING THEIR HANDS CLEAN? What s Included with Your Nexa Hand Sanitizer Station Order: 80 One white Nexa Classic of all
Ecolab Total Hand Hygiene System Clean without a doubt. WHY AREN T YOUR EMPLOYEES GETTING THEIR HANDS CLEAN? What s Included with Your Nexa Hand Sanitizer Station Order: 80 One white Nexa Classic of all
United States Patent (19)
 United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
PRODUCT SELECTION AND INGREDIENTS Date:
 11 PRODUCT SELECTION AND INGREDIENTS Date: TOPIC 1: PRODUCTS Rating: Text pages: 224 257 1 Name the five main categories in skin care products (cleansers) (exfoliates) (masks) (toners) (treatment creams
11 PRODUCT SELECTION AND INGREDIENTS Date: TOPIC 1: PRODUCTS Rating: Text pages: 224 257 1 Name the five main categories in skin care products (cleansers) (exfoliates) (masks) (toners) (treatment creams
EcoHydra Antimicrobial Hand Lotion. Product Overview. Physical Properties. Product Description. Regulatory Compliance. Key Features and Benefits
 EcoHydra Antimicrobial Hand Lotion Product Overview Product Description The EcoHydra Antimicrobial Hand Lotion is a daily moisturising lotion that helps heal dry or chapped skin whilst its antimicrobial
EcoHydra Antimicrobial Hand Lotion Product Overview Product Description The EcoHydra Antimicrobial Hand Lotion is a daily moisturising lotion that helps heal dry or chapped skin whilst its antimicrobial
PO Box 5411 Arlington, TX SF A-348
 SF A-348 PRODUCT DESCRIPTION SF A-348 organosilicone is a proprietary copolymer that represents a class of aminosilicone polyalkyleneoxide copolymers for hair conditioning. Conventional organomodified
SF A-348 PRODUCT DESCRIPTION SF A-348 organosilicone is a proprietary copolymer that represents a class of aminosilicone polyalkyleneoxide copolymers for hair conditioning. Conventional organomodified
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
Contents. I. Sweaty and Smelly Feet... 3 II. How Can We Prevent Smelly Feet... 5 III. 10 Simple Cures for Smelly Feet... 8 IV. Final thoughts...
 ~ 1 ~ Contents I. Sweaty and Smelly Feet... 3 II. How Can We Prevent Smelly Feet... 5 III. 10 Simple Cures for Smelly Feet... 8 IV. Final thoughts... 17 ~ 2 ~ I. Sweaty and Smelly Feet Every foot has its
~ 1 ~ Contents I. Sweaty and Smelly Feet... 3 II. How Can We Prevent Smelly Feet... 5 III. 10 Simple Cures for Smelly Feet... 8 IV. Final thoughts... 17 ~ 2 ~ I. Sweaty and Smelly Feet Every foot has its
(12) United States Patent (10) Patent No.: US 6,582,709 B1
 USOO82709B1 (12) United States Patent (10) Patent No.: Maor et al. () Date of Patent: Jun. 24, 2003 (54) CREAM COMPOSITION COMPRISING (56) References Cited DEAD SEA MUD U.S. PATENT DOCUMENTS (75) Inventors:
USOO82709B1 (12) United States Patent (10) Patent No.: Maor et al. () Date of Patent: Jun. 24, 2003 (54) CREAM COMPOSITION COMPRISING (56) References Cited DEAD SEA MUD U.S. PATENT DOCUMENTS (75) Inventors:
(*) Notice: Slity sight 58 $5 E. G.
 USOO629.7208B1 (12) United States Patent (10) Patent No.: US 6,297,208 B1 Crist () Date of Patent: Oct. 2, 2001 (54) RUST STAIN REMOVAL FORMULA 4,891,0 1/1990 Gross et al.. 4,963,233 * 10/1990 Mathew...
USOO629.7208B1 (12) United States Patent (10) Patent No.: US 6,297,208 B1 Crist () Date of Patent: Oct. 2, 2001 (54) RUST STAIN REMOVAL FORMULA 4,891,0 1/1990 Gross et al.. 4,963,233 * 10/1990 Mathew...
PATENT APPLICATION. int. ci.5: A61K 31/035, A61K7/06
 Europaisches Patentamt 19 European Patent Office Office europeen des brevets Publication number : 0 467 660 A2 EUROPEAN PATENT APPLICATION Application number : 91306490.3 int. ci.5: A61K 31/035, A61K7/06
Europaisches Patentamt 19 European Patent Office Office europeen des brevets Publication number : 0 467 660 A2 EUROPEAN PATENT APPLICATION Application number : 91306490.3 int. ci.5: A61K 31/035, A61K7/06
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
(19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
6/23/2010 Naturally Pure ~ Anxiety Free1
 Melody Thacker, Independent Watkins Associate ID # 323239 To learn more, request a catalog or service or to place an order Call Toll Free: 1-866-452-6948 Visit: http://www.watkinsonline.com/thacker 6/23/2010
Melody Thacker, Independent Watkins Associate ID # 323239 To learn more, request a catalog or service or to place an order Call Toll Free: 1-866-452-6948 Visit: http://www.watkinsonline.com/thacker 6/23/2010
AneStop Prensentation
 AneStop Prensentation DERMICA LABORATOIRES AG Reitergasse nº1, 2nd floor. 8004. Zurich. Switzerland. info@dermica.ch T:+41435080698 DERMICA LABORATOIRES EUROPE S.L Avd. Ciclista Mariano Rojas 76. 5ª planta.
AneStop Prensentation DERMICA LABORATOIRES AG Reitergasse nº1, 2nd floor. 8004. Zurich. Switzerland. info@dermica.ch T:+41435080698 DERMICA LABORATOIRES EUROPE S.L Avd. Ciclista Mariano Rojas 76. 5ª planta.
DECON-HAND. Instant Hand Sanitizer. HAND_VL Revised 19 November, Technical Data File
 Instant Hand Sanitizer HAND_VL-1401-3 Revised 19 November, 2013 Technical Data File, USA T: 610.644.8335 F: 610.644.8336 www.sterile.com 1 of 7 PRODUCT DESCRIPTION VAI manufactures an alcohol-based hand
Instant Hand Sanitizer HAND_VL-1401-3 Revised 19 November, 2013 Technical Data File, USA T: 610.644.8335 F: 610.644.8336 www.sterile.com 1 of 7 PRODUCT DESCRIPTION VAI manufactures an alcohol-based hand
(12) United States Patent (10) Patent No.: US 7.462,753 B2. Ma et al. (45) Date of Patent: Dec. 9, 2008
 USOO7462753B2 (12) United States Patent (10) Patent No.: Ma et al. (45) Date of Patent: Dec. 9, 2008 (54) NANO-SILVER WOUND DRESSING 4,529.623 A * 7/1985 Maggs... 427,227 4.928,681 A * 5/1990 Langston
USOO7462753B2 (12) United States Patent (10) Patent No.: Ma et al. (45) Date of Patent: Dec. 9, 2008 (54) NANO-SILVER WOUND DRESSING 4,529.623 A * 7/1985 Maggs... 427,227 4.928,681 A * 5/1990 Langston
Raspberry/White Peach Facial Protocol
 Raspberry/White Peach Facial Protocol Raspberries, White Peach and a Winter Complex come together to gently exfoliate and nourish/hydrate your skin through the use of (1) the DermaPep peptide with retinoic
Raspberry/White Peach Facial Protocol Raspberries, White Peach and a Winter Complex come together to gently exfoliate and nourish/hydrate your skin through the use of (1) the DermaPep peptide with retinoic
(12) United States Patent
 (12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
(12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
Int. Cl."... F21V1/06 U.S. C /352; 362/358. References Cited U.S. PATENT DOCUMENTS 3,787,676 l/1974 Korach /352
 United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
United States Patent (19)
 USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O18O194A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180194 A1 Cutter (43) Pub. Date: Jul.19, 2012 (54) GARMENTS WITH ADJUSTABLE WAISTS (52) U.S. Cl.... 2/237
(19) United States US 2012O18O194A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180194 A1 Cutter (43) Pub. Date: Jul.19, 2012 (54) GARMENTS WITH ADJUSTABLE WAISTS (52) U.S. Cl.... 2/237
FLORAESTERS CHEMISTRY
 Bulletin 3 FLORAESTERS 60 FLORAESTERS CHEMISTRY Natural jojoba oil is actually not an oils but an array of oxidatively stable, liquid wax esters. The dominant molecular structure is a straight chain monoester
Bulletin 3 FLORAESTERS 60 FLORAESTERS CHEMISTRY Natural jojoba oil is actually not an oils but an array of oxidatively stable, liquid wax esters. The dominant molecular structure is a straight chain monoester
United States Patent (19) Humbrecht
 United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
12) United States Patent 10) Patent No.: US 6,433,024 B1
 USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
(12) United States Patent (10) Patent No.: US 6,422,036 B1. Giannis et al. (45) Date of Patent: Jul. 23, 2002
 USOO6422036B1 (12) United States Patent (10) Patent No.: US 6,422,036 B1 Giannis et al. (45) Date of Patent: Jul. 23, 2002 (54) JEWELRY CLASP 4,611,368 9/1986 Battersby... 24/116 R 5,214,940 A * 6/1993
USOO6422036B1 (12) United States Patent (10) Patent No.: US 6,422,036 B1 Giannis et al. (45) Date of Patent: Jul. 23, 2002 (54) JEWELRY CLASP 4,611,368 9/1986 Battersby... 24/116 R 5,214,940 A * 6/1993
Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents
 Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents Sharing expertise around the world As a global manufacturer of cosmetic ingredients and functional fine chemicals, Hannong Chemicals
Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents Sharing expertise around the world As a global manufacturer of cosmetic ingredients and functional fine chemicals, Hannong Chemicals
United States Patent (19)
 United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
Main ready-made products list [Quasi drugs] Skin-whitening series Product names Active ingredients Product characteristics
![Main ready-made products list [Quasi drugs] Skin-whitening series Product names Active ingredients Product characteristics Main ready-made products list [Quasi drugs] Skin-whitening series Product names Active ingredients Product characteristics](/thumbs/96/126828594.jpg) [Quasi drugs] Skin-whitening series Product names Active ingredients Product characteristics Medicated white lotion TR Double effective for whitening and anti-inflammation ingredients Paraben-free, Synthetic
[Quasi drugs] Skin-whitening series Product names Active ingredients Product characteristics Medicated white lotion TR Double effective for whitening and anti-inflammation ingredients Paraben-free, Synthetic
Arctic Cranberry Wintermint Facial Protocol
 Arctic Cranberry Wintermint Facial Protocol This wintery facial contains an arctic cranberry enzyme with 2% mandelic, 2% lactic, 2% arbutin and 2% kojic which is an exceptional combination for an extreme
Arctic Cranberry Wintermint Facial Protocol This wintery facial contains an arctic cranberry enzyme with 2% mandelic, 2% lactic, 2% arbutin and 2% kojic which is an exceptional combination for an extreme
Product Information n File (PIF) ) Summary
 Product Information n File (PIF) ) Summary ma 1. Product Description Product name Green Tea Anti-ageinuse of the pro oduct Frame formulation: 1.11 Composition type: Cream General purp pose: Face mas sk
Product Information n File (PIF) ) Summary ma 1. Product Description Product name Green Tea Anti-ageinuse of the pro oduct Frame formulation: 1.11 Composition type: Cream General purp pose: Face mas sk
Surfactants Soaps Detergents
 Surfactants Soaps Detergents wikipedia Surface tension = forces on molecules at a liquid air interface are different from those felt by molecules in the bulk liquid Surface tension keeps objects with higher
Surfactants Soaps Detergents wikipedia Surface tension = forces on molecules at a liquid air interface are different from those felt by molecules in the bulk liquid Surface tension keeps objects with higher
Patented BCX-CA. (Natural anti-acne treatment)
 Patented BCX-CA (Natural anti-acne treatment) Product introduction BCX-CA (Natural anti-acne treatment) 1. A n t i - a c n e t r e a t m e n t t h a t m a n u f a c t u r e d w i t h n a t u r a l i n
Patented BCX-CA (Natural anti-acne treatment) Product introduction BCX-CA (Natural anti-acne treatment) 1. A n t i - a c n e t r e a t m e n t t h a t m a n u f a c t u r e d w i t h n a t u r a l i n
Addition of Benzylic Bromines to Ethyl Paraben. been found in studies to bind to the estrogen receptors. Because of this binding site, many
 Harrison Kish and Caitlin Harris December 1, 2017 Organic Chemistry II Addition of Benzylic Bromines to Ethyl Paraben Abstract Parabens are chemical compounds that have antibacterial properties and are
Harrison Kish and Caitlin Harris December 1, 2017 Organic Chemistry II Addition of Benzylic Bromines to Ethyl Paraben Abstract Parabens are chemical compounds that have antibacterial properties and are
Influence of Clear Liquid Shampoo Components on Preservative Activity of Parabens
 Influence of Clear Liquid Shampoo Components on Preservative Activity of Parabens Suwipa Sree-iam, Sanae Kaewnopparat and Sirirat pinsuwan. Faculty of Pharmaceutical Sciences, Pharmaceutical Technology
Influence of Clear Liquid Shampoo Components on Preservative Activity of Parabens Suwipa Sree-iam, Sanae Kaewnopparat and Sirirat pinsuwan. Faculty of Pharmaceutical Sciences, Pharmaceutical Technology
YOUR CLEAR CHOICE FOR PREMIUM PACKAGING SOLUTIONS
 YOUR CLEAR CHOICE FOR PREMIUM PACKAGING SOLUTIONS Call: 844-766-7819 Email: info@saxco.com Visit: www.saxco.com Acids: MINERALS: UP TO 5% ONLY: Hydrochloric P/SCKLF*** Higher concentration use Nitric P/SCKLF***
YOUR CLEAR CHOICE FOR PREMIUM PACKAGING SOLUTIONS Call: 844-766-7819 Email: info@saxco.com Visit: www.saxco.com Acids: MINERALS: UP TO 5% ONLY: Hydrochloric P/SCKLF*** Higher concentration use Nitric P/SCKLF***
(12) United States Patent (10) Patent No.: US 9.407, B2
 USOO9407742B2 (12) United States Patent (10) Patent No.: US 9.407,742 9 9 B2 NOble Nava (45) Date of Patent: Aug. 2, 2016 (54) CELL PHONE HOLSTER 2005/008 (2013.01); A45F 2200/0516 (2013.01); H04B 2001/3861
USOO9407742B2 (12) United States Patent (10) Patent No.: US 9.407,742 9 9 B2 NOble Nava (45) Date of Patent: Aug. 2, 2016 (54) CELL PHONE HOLSTER 2005/008 (2013.01); A45F 2200/0516 (2013.01); H04B 2001/3861
Chemistry of hair and beauty products
 Chemistry of hair and beauty products UHB157 H/508/0606 Learner name: VRQ Learner number: VTCT is the specialist awarding organisation for the Hairdressing, Beauty Therapy, Complementary Therapy, Hospitality
Chemistry of hair and beauty products UHB157 H/508/0606 Learner name: VRQ Learner number: VTCT is the specialist awarding organisation for the Hairdressing, Beauty Therapy, Complementary Therapy, Hospitality
Item/Package Details Size Item Bottle ph Shelf Life 1.0 oz/29.6 ml 1101 Lucite Matte Silver Pump months
 B-Kleer 10 Lotion Use of mild to moderate acne. Gently exfoliates encrustation and acne pustules. Penetrates pores to eliminate most acne pimples. Reduces the severity of acne blemishes. Help prevent new
B-Kleer 10 Lotion Use of mild to moderate acne. Gently exfoliates encrustation and acne pustules. Penetrates pores to eliminate most acne pimples. Reduces the severity of acne blemishes. Help prevent new
Chlorhexidine Gluconate Antimicrobial Skin Cleansers TECHNICAL DATA
 Chlorhexidine Gluconate Antimicrobial Skin Cleansers Manufactured for: Aplicare, Inc. 550 Research Parkway Meriden, CT 06450 Tel: 800-760-3236 Fax: 203-630-4876 Web: www.cloroxhealthcare.com BACKGROUND
Chlorhexidine Gluconate Antimicrobial Skin Cleansers Manufactured for: Aplicare, Inc. 550 Research Parkway Meriden, CT 06450 Tel: 800-760-3236 Fax: 203-630-4876 Web: www.cloroxhealthcare.com BACKGROUND
TECHNICAL DATA SHEET
 TECHNICAL DATA SHEET Barrier cream for protection against water and non-water based contaminants AREA OF APPLICATION: Effective Protection against non-water based contaminants (ex., petroleum products,
TECHNICAL DATA SHEET Barrier cream for protection against water and non-water based contaminants AREA OF APPLICATION: Effective Protection against non-water based contaminants (ex., petroleum products,
Jake Rocchi CCHS, 9 th grade 1 st year in PJAS. Bleach Effects on Microbial Life
 Jake Rocchi CCHS, 9 th grade 1 st year in PJAS Bleach Effects on Microbial Life Clorox Bleach Ingredients Sodium hypochlorite Sodium chlorite Sodium carbonate Sodium hydroxide Sodium polycarbonate Effective
Jake Rocchi CCHS, 9 th grade 1 st year in PJAS Bleach Effects on Microbial Life Clorox Bleach Ingredients Sodium hypochlorite Sodium chlorite Sodium carbonate Sodium hydroxide Sodium polycarbonate Effective
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
Developed by Western Massachusetts Coalition for Occupational Safety and Health. Funded by The Toxics Use Reduction Institute
 Developed by Western Massachusetts Coalition for Occupational Safety and Health Funded by The Toxics Use Reduction Institute Hair Relaxing Process Health and Safety Concerns Health and Safety Precautions
Developed by Western Massachusetts Coalition for Occupational Safety and Health Funded by The Toxics Use Reduction Institute Hair Relaxing Process Health and Safety Concerns Health and Safety Precautions
Cherry Chocolate Facial Protocol
 Cherry Chocolate Facial Protocol Love is in the air with this made-for-each-other cherry and chocolate duo. This perfect couple will warm your client s skin and tantalize with the indulgent scents of cherry
Cherry Chocolate Facial Protocol Love is in the air with this made-for-each-other cherry and chocolate duo. This perfect couple will warm your client s skin and tantalize with the indulgent scents of cherry
