Working at Biosafety Level 2 (BSL-2)
|
|
|
- Joshua Tyler
- 5 years ago
- Views:
Transcription
1 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents and potentially infectious agents at Biosafety Level 2 (BSL- 2) is aware of the risks and the measures that are in place to protect them and avoid exposure to these materials. 2.0 Scope This procedure applies to all individuals, including Principal Investigators (PIs), researchers, instructors, laboratory/clinical managers, students, other personnel, and visitors who handle infectious or potentially infectious materials at a Biosafety Level 2 (BSL-2). It covers engineering and work controls, personal protective equipment (PPE), and facility requirements for handling these types of materials. 3.0 Definitions Biosafety Level - Designation which describes laboratory practices and techniques, safety equipment and laboratory facilities appropriate for the materials in use and the operations performed. Biosafety Level 1 (BSL-1) - A laboratory level suitable for work involving wellcharacterized agents not known to consistently cause disease in immunocompetent adult humans. These agents present minimal potential hazard to laboratory personnel and the environment. Biosafety Level 2 (BSL-2) - A laboratory level suitable for work involving agents of moderate potential hazard to personnel and the environment. It includes various bacteria and viruses that cause disease in humans for which there is often a vaccine or treatment available or potential plant pests and animal pathogens where accidental release to the environment could have serious effects on agriculture and/or environment. Agents handled at BSL-2 often cause common childhood diseases, and are not usually spread via the airborne route in a lab setting, such as C. difficile, Staph aureus, Salmonella, etc. Potential biohazards and Other Potentially Infectious Materials (OPIM) are also handled at BSL-2. Materials handled at BSL-2 are generally safe to work with if using standard microbiological practices, such as hand washing, wearing gloves, preventing aerosols, etc. ISSUE DATE: DD/MONTH/YY Page 1 of 20
2 Biohazard A biological agent or substance that can cause disease in humans or animals. These include, but are not limited to, infectious organisms and parasitic agents, biological toxins, infected animals, infectious clinical specimens, and/or equipment contaminated with infectious agents. A biohazard may be handled at BSL-2, 3, or 4, depending on the severity of the disease it causes, how it is transmitted, the quantities being handled, and the types of operations to be done, etc. Bloodborne Pathogens - Pathogenic microorganisms that are present in human blood and can cause disease in humans. These pathogens include, but are not limited to, hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV). BSL-2 Biohazard Signage - Includes the international biohazard symbol, the word Biohazard and shall be fluorescent orange-red or predominantly so, with lettering and symbols in a contrasting color, and include a list of the materials in use, contact information, and any special entry requirements or warnings. Engineering Controls - Includes devices/equipment (e.g., biological safety cabinet (BSC), sharps containers) that contain or minimize the biological hazards in the workplace. Human cell lines Immortalized cells propagated in vitro from primary explants of human tissue or body fluid. ATCC and OSHA recommend working with human cell lines as if potentially infectious because they are only tested for certain adventitious agents since there are no tests available for all possible contaminants and because some lines are only tested by the depositor. Non-hazardous material - Generally includes animal specimens and animal cell culture lines (hamster, mouse, etc.) unless these are known to be infected with a pathogen. Other Potentially Infectious Materials (OPIM) - (1) The following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visibly contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids; (2) Any unfixed tissue or organ (other than intact skin) from a human (living or dead); and (3) HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV. Pathogen - For shipping purposes, microorganisms (including bacteria, viruses, parasites, fungi) and other agents such as prions, which can cause disease in humans or animals. ISSUE DATE: DD/MONTH/YY Page 2 of 20
3 Reasonably Expected to Contain a Pathogen - For shipping purposes, this refers to a material that has been tested and found to be or contain a pathogen, or that has been taken from a patient known to be infected with a pathogen. Universal Precautions - Refers to the basic biosafety practices for handling all blood, body fluids and cell lines from humans or non-human primates as potentially infectious. 4.0 Responsibilities 5.0 Procedure U of L employees, students, visitors and contract workers who work with biohazardous or potentially biohazardous materials are responsible for compliance with this procedure. U of L personnel in supervisory or management positions (e.g., PIs, Lab Managers or other managers) are responsible for ensuring that employees and/or students are adequately trained to perform their duties safely in keeping with this procedure. The Biosafety Officer (BSO) is responsible for coordinating technical support for the implementation of this procedure and updating this procedure as needed. 5.1 Facility requirements for BSL-2 areas The laboratory is designed so that it can be easily cleaned. Carpeting and rugs are not allowed in the facility. Chairs and other furniture must be covered with a non-porous material that can be easily cleaned and decontaminated with a disinfectant. Laboratory doors should be self-closing and have locks. Work surfaces should be impervious to water and resistant to acids, alkalis, organic solvents and moderate heat. The laboratory/production area should be kept neat, clean and free of materials not pertinent to the work. Each laboratory must contain a sink for hand washing and an eyewash station. Sinks may be manually, hands-free, or automatically operated. It should be located near the exit door if possible. If a sink with running water is not immediately available, waterless hand sanitizer must be provided. Employees must then be instructed to wash hands with soap and water as soon as possible. Furniture must be sturdy and placed so that spaces between furniture and equipment are accessible for cleaning. ISSUE DATE: DD/MONTH/YY Page 3 of 20
4 A method for decontamination of infectious materials is available. Windows that can be opened must be fitted with a fly screen. When working with biohazardous materials, Biological Safety Cabinets (BSCs) should be provided for containment of aerosol generating procedures. Employees are trained on the proper use of the BSC with more information provided in the Working Safely in a Biosafety Cabinet (BSC). An insect and rodent control program is in effect. 5.2 Procedural Requirements Access to the laboratory is limited to trained or escorted personnel. At BSL-2, persons at increased risk of infection or for whom infection may be unusually hazardous should be aware of the hazards present (via training and/or door signage). The entrance to the laboratory must be identified with the appropriate biohazard signage, which lists the biohazardous materials in use and the names and phone number(s) of personnel to contact in case of an emergency, and any special entry requirements. At BSL-2, doors must be closed when work with biohazardous material is in progress. The laboratory supervisor must ensure that laboratory personnel demonstrate proficiency in standard and special microbiological practices before working with BSL- 2 agents. The laboratory supervisor must ensure that laboratory personnel receive appropriate training regarding their duties, the necessary precautions to prevent exposures, and exposure evaluation procedures. Personnel must receive annual updates if working with human-sourced materials (Bloodborne Pathogen Training) or additional training when procedural or policy changes occur. Appropriate training may be found on the DEHS website. Laboratory personnel must be provided medical surveillance, as appropriate, and offered available immunizations for agents handled or potentially present in the laboratory. Personnel who work with human sourced materials or human cell lines should be aware of the HBV vaccination program and who to contact regarding any health issues. ISSUE DATE: DD/MONTH/YY Page 4 of 20
5 Employees are advised to notify the medical provider or their personal physician to discuss any significant change (e.g., pregnancy, immune suppressed condition) in their health status. A laboratory-specific biosafety manual must be prepared and adopted as policy for the lab. The biosafety manual must be available and accessible. This manual will contain standard operational procedures as well as equipment-specific and laboratory-specific procedures. Laboratory coats or gowns must be worn over street clothes when working with biohazardous/potentially biohazardous materials or in the same area where these materials are handled. o The lab coats should not be worn outside of the laboratory, i.e., they shall be removed before entering offices, conference rooms, break rooms, restrooms, etc. Protective lab clothing that has been used in the lab must not be stored in the same lockers as street clothing, and must not be taken home for laundering. o If a cloth lab coat laundering system is not in place, disposable lab coats will be used. All lab coats should be changed on a regular basis. o If an outside laundry service is used for lab coats, soiled lab coats will be collected near where they were used and placed in bags/containers marked SOILED LAUNDRY, USE UNIVERSAL PRECAUTIONS. Laundry will not be handled or sorted at the location of use. o If potentially infectious material is spilled on a cloth reusable coat, it should immediately be removed and treated with a disinfectant, e.g., apply an approved disinfectant for a minimum of 10 minutes, then rinse the coat with water and place it in the soiled laundry bin. Contaminated disposable lab coats must be carefully removed, so as not to contaminate the user, and placed in the solid biohazard waste bin. Waterproof gloves must be worn for all procedures that involve contact with potentially infectious material, or surfaces or equipment that may be contaminated with these materials. It is advisable to double glove when working in some situations (e.g., inside the BSC with potentially infectious material). o Gloves must be changed immediately if contaminated or damaged. Wash hands with soap and water any time gloves are changed. o After use, gloves should be removed using a technique to prevent hand contamination. o Disposable gloves may not be washed or reused. o Alternatives to latex gloves are provided; hypoallergenic gloves, glove liners, and powder-free gloves are available for those with allergies. Various sizes of gloves are provided in the work area. ISSUE DATE: DD/MONTH/YY Page 5 of 20
6 o If a rash or reactions develops after wearing gloves, switch to a different brand or type of gloves. If the rash persists, consult with Campus Health Services. Safety glasses should be worn whenever an employee is handling potentially infectious materials or chemicals, including while working in the BSC. Full face shields or goggles and mucous membrane protection, e.g., a molded face mask or equivalent (see Appendix E), must be worn when performing procedures that may involve splashing, spraying, spattering, or generation of droplets. Employees wearing contact lenses may have additional risk from exposure to chemicals (e.g., strong acids) and should consider wearing goggles for certain tasks. Open-toed footwear or sling-back or open-back shoes must not be worn in laboratories. Closed heel toe and closed footwear is recommended. Flip-flops are unsafe and are strictly prohibited. Persons must wash their hands after handling potentially infectious materials, immediately after removing gloves, and when leaving the work area. If a sink with running water is not immediately available in a work area, a waterless hand sanitizer must be provided for employees. Employees must then be instructed to wash their hands with soap and water as soon as possible. Mouth pipetting is prohibited; mechanical devices must be used. Eating, drinking, storing food, smoking, and applying cosmetics or lip balm, or handling contact lenses are not allowed in the laboratory or adjacent desk area. Food may be stored in cabinets or refrigerators designated solely for this purpose outside of the work area. Laboratory materials/equipment must not be placed in the mouth. Written documents that are expected to be removed from the work area need to be protected from contamination while in the lab, e.g., while in use, documents are kept on separate clean work surface, documents are kept in a plastic document holder, etc. All procedures must be performed carefully to minimize the creation of aerosols. Aerosol generating procedures (e.g., blending, sonicating, vortexing, etc.) must be handled in a BSC or other containment device. o Additional personal protective equipment, which may include a shield or goggles and mucous membrane protection, e.g., a molded face mask or equivalent, may also be used for procedures that do not fit into a BSC or other containment device. The use of sharps and glassware should be minimized. o Needles/ syringes or other sharps should only be used where no alternatives are available. Sharps with engineered sharps injury protection must be evaluated and ISSUE DATE: DD/MONTH/YY Page 6 of 20
7 substituted where possible. This evaluation should be conducted annually and be documented. In situations where sharps must continue to be used, additional personal protective equipment and/or work practices should be specified. Input shall be solicited from all users in the selection of safer devices and work practices. o The use of syringes with hypodermic needles attached should be limited to phlebotomy, piercing a septum or animal work. Where used, only luer-lock or onepiece disposable needle-syringe units should be used. Needles must not be bent, sheared, or recapped. They should be disposed of in a puncture resistant container readily accessible to the work area immediately following use. These sharps containers are never overfilled; they are used in the upright position and closed and sealed when the contents near the full line on the container. o Plasticware should be substituted for glass wherever possible, e.g., blood tubes, capillary tubes, Pasteur pipets, centrifuge tubes, etc. Use mechanical means (forceps, dustpan and brush, cardboard shovel ) to remove broken contaminated glassware and dispose of into a puncture resistant container. Never handle broken glass with the hands, even if wearing gloves. Animal and plants not associated with the work being performed are not permitted in the laboratory. When centrifuges are used for processing potentially infectious or potentially infectious materials, they must have sealed heads or be used with safety cups if the unit is not enclosed in a containment device. If possible, cups/rotors are loaded/unloaded inside the BSC in case of leakage. When a vacuum is used, the lines must be protected with a liquid disinfectant trap and an in-line HEPA. (See Appendix B of this document). All potentially biohazardous waste must be disposed of in accordance with local, state and federal regulations. Where waste needs to be transported for treatment, it must be appropriately packaged and labeled. Consult Appendix A of this document for additional information on disposal of laboratory waste. o Solid Waste - Generally, contaminated solid waste is any waste that has been in contact with biohazardous or potentially biohazardous material. This includes, but is not limited to, the following: vials, centrifuge tubes, gloves, paper towels, spill cleanup materials, disposable lab coats, etc. Consult Appendix A of this document for U of L waste disposal procedures. i. It is recommended that plastic/glass serological pipets be deposited into a pipet keeper box or cardboard box, so as not to puncture the biohazard bags. Also, pipet tips, blood tubes, etc. should be deposited into a container (Benchtop keeper, plastic jug or bottle, etc.) that will prevent them from breaking or ISSUE DATE: DD/MONTH/YY Page 7 of 20
8 puncturing the biohazard bags. If using a plastic bottle or jug for collection, it must have a biohazard label. ii. This type of waste is autoclaved/disposed off-site by a contracted vendor. Labs must use vendor supplied red bags and biohazardous marked box, tote, or red barrel. Labs must also mark building name, room number, and contact name and phone on each full container. Only 2 inch wide clear tape can be used to secure boxes. iii. NOTE: if solid waste can be effectively decontaminated by soaking/rinsing with bleach or other approved disinfectant, it is no longer considered contaminated and does not need to be discarded into the biohazard waste bag. o Sharps i. Because of the hazard posed by contaminated sharps, broken glass, Pasteur pipettes, needles, etc., these materials must be placed in a puncture-resistant, leak-proof container, prior to disposal into a biohazard waste bag. The puncture-resistant container must be marked with a biohazard symbol and the word Biohazard. The container should be readily accessible to the user, kept in the upright position, and replaced when full (as indicated by the container labeling). o Liquids i. Small quantities of liquid (such as those in a vial or tube) can usually be safely placed in the biohazard solid waste bag. Larger quantities of potentially infectious liquid can be treated with a liquid disinfectant. During collection, the liquid waste collection container must be labeled with the biohazard symbol and the word Biohazard. Sufficient absorbent material should be placed around the container to absorb liquid in the event of accidental release during transport. ii. Bulk liquids can also be decontaminated with bleach or Wescodyne (see Selection and Use of Disinfectants). Containers of infectious material must be appropriately labeled with a biohazard label containing the international biohazard symbol, the word Biohazard, with lettering and symbols in a contrasting color: o Where not possible to label each container, the units must be placed in a marked carrier. o Refrigerators, liquid nitrogen tanks or freezers used to store these materials must be marked with a biohazard label. Incubators used with potentially biohazardous materials (e.g., human tissue culture), must be labeled with a biohazard label. o Containers of material being transported from one lab to another must be placed in a sealed bag, covered carrier, or other suitable secondary container. ISSUE DATE: DD/MONTH/YY Page 8 of 20
9 Clean and disinfect work surfaces at least once a day, and after any spill, using an appropriate disinfectant. See Selection and Use of Disinfectants for a list of disinfectants. Covers or drapes used on work surfaces must be disposed of after use. All cuts or abrasions on potentially exposed skin, especially hands and forearms, must be covered with an occlusive, i.e., waterproof bandage. Skin conditions like dermatitis, psoriasis, etc., need to be similarly protected. If it is not feasible to protect the affected area, the individual should notify their supervisor prior to proceeding. All contaminated instruments and equipment must be decontaminated prior to servicing, disposal, or shipping. Follow the manufacturers directions if available. If not, the unit should be emptied of all potentially infectious materials. The unit should be wiped down with a detergent solution followed by a wipe down with an approved disinfectant. Label decontaminated equipment with an Equipment Decontamination Verification Tag (see Appendix C of this document) or other appropriate marking system. Note any areas of the equipment that could not be decontaminated. For biosafety cabinet decontamination procedures, refer to Working Safely in a Biosafety Cabinet (BSC) for BSL-2. Contaminated reusables (e.g., glassware, plastic ware, stainless steel, etc.) should be rinsed with an appropriate disinfectant after use and before washing. Spills and accidents which result in overt or potential exposure to potentially infectious agents must be reported to the supervisor and/or PI. Additionally, the University of Louisville s Biological Safety Officer should be notified. The incident must be documented on an Biological Exposure Incident Follow-up Survey (see Appendix D of this document). 5.3 General Spill Cleanup Procedure Spills of infectious/potentially infectious materials must be cleaned up by the laboratory users, who are trained in the process. Spill cleanup materials, such as disinfectant, an aqueous detergent, paper towels, etc. should be available in the laboratory. Consult Selection and Use of Disinfectants for a list of detergents and disinfectants. o For small spills, wipe the area with a paper towel discard into the biohazard waste container. Wipe the area again with an aqueous detergent, followed by wiping with a disinfectant. o If the disinfectant contains detergent (e.g., Cavicide) the same product can be used for pre-cleaning and disinfecting. o Discard all materials into the biowaste container. o If the spill is large, avoid contaminating surrounding areas by decontaminating or removing contaminated shoes or clothing as soon as is feasible. ISSUE DATE: DD/MONTH/YY Page 9 of 20
10 o If an individual has any exposed skin or mucous membrane contact with the potentially biohazardous material, wash or rinse the affected area immediately and seek medical attention. Alert others about the spill to arrange for cleanup. o Change gloves/lab coat if contaminated: don gloves, safety glasses and a lab coat if not already wearing them. o Carefully soak up the bulk of the liquid with absorbent material (paper towels, absorbent pads, etc.). Since most disinfectants are less effective in the presence of organic materials, e.g., media with serum, the bulk of the spill should be absorbed prior to disinfection. o Dispose of all contaminated materials in a biowaste bag. o Clean the spill of all visible material using an aqueous detergent such as Alconox, 7X, Vesphene, Cavicide, etc. If the disinfectant contains detergent (e.g., Cavicide) the same product can be used for pre-cleaning and disinfecting. o To disinfect, wipe the spill site using an approved disinfectant, such as 0.1% sodium hypochlorite, Cavicide, etc. o Clean and disinfect any furniture or equipment that may have been contaminated in the spill. 5.4 Large spills of ~ 500 ml or more of biohazardous or other potentially infectious materials o Avert your face and move away from the area to avoid breathing any aerosols that may have been generated by the spill. In dealing with large spills of potentially infectious material, contain the spread of the spill with absorbent diking material, if possible. o If an individual has any exposed skin or mucous membrane contact with the material, they must immediately wash or rinse the affected area and seek medical attention as soon as possible. They should alert others about the spill so that appropriate quarantine and cleanup procedure can be followed. o If the spill occurred in a laboratory, leave the room and evacuate other personnel. Post signs if necessary. If the spill occurred in an open area or in a corridor, evacuate the personnel in the immediate area and block off the area to prevent others from entering until the cleanup is complete. o Wait approximately 30 minutes for aerosols to dissipate before returning to spill area to initiate cleanup. o Wear a mask, lab coat, goggles, and waterproof gloves for cleanup. If there is a potential for contaminating the shoes, rubber or waterproof protective shoe coverings should be worn. o For non-radioactive spills, carefully pour undiluted chlorine bleach around the perimeter of the spill, allowing it to flow into the middle of the spill. If the spill is very large, use a long-handled squeegee or similar item to carefully push the disinfectant to the center of the area. Allow at least 15 minutes of contact time. o Carefully soak up the bulk of the liquid with absorbent material (paper towels, absorbent pads, etc.). Since most disinfectants are less effective in the presence of ISSUE DATE: DD/MONTH/YY Page 10 of 20
11 organic materials, e.g., media with serum, the bulk of the spill should be absorbed prior to disinfection. o Dispose of all contaminated materials in a biowaste bag. o Clean the spill of all visible material using an aqueous detergent such as Alconox, 7X, Vesphene, Cavicide, etc. If the disinfectant contains detergent (e.g., Cavicide) the same product can be used for pre-cleaning and disinfecting. o To disinfect, wipe the spill site using an approved disinfectant, such as 0.1% sodium hypochlorite, Cavicide, etc. o Clean and disinfect any furniture or equipment that may have been contaminated in the spill. o Report all large spills to the Biosafety Office. 5.5 Spills involving sharps or concentrated infectious materials o Avert your face and move away from the area to avoid breathing any aerosols that may have been generated by the spill. Avoid contaminating surrounding areas by decontaminating or removing contaminated shoes or clothing as soon as is feasible. o Disinfect the spill first by covering the spill with paper towels or other absorbent material, and then carefully saturate the area with a 10% or stronger bleach solution or other appropriate approved liquid disinfectant (Cavicide, etc.). o Allow the disinfectant at least 15 minutes of contact time on the spill. o Use tongs, forceps cardboard pushers or some other mechanical means to remove sharps and place in a biohazard sharps container. DO NOT USE HANDS. o Carefully soak up the bulk of the liquid with absorbent material (paper towels, absorbent granular material or silica gel). Care should be taken when soaking up the liquid, since glass shards may still be present. Since most disinfectants are less effective in the presence of organic materials, e.g., broth, etc., the bulk of the spill should be absorbed prior to disinfection. o Dispose of all contaminated materials in a biowaste bag. o Clean the spill of all visible material using an aqueous detergent such as Alconox, 7X, Vesphene, etc. o To disinfect, wipe the spill site using an approved disinfectant, such as 0.1% sodium hypochlorite, Cavicide, etc. For a complete list of approved disinfectants, see the Selection and Use of Disinfectants. o Clean and disinfect any furniture or equipment that may have been contaminated in the spill. 5.5 First Aid for Accidental Exposures Persons exposed to biohazardous or potentially biohazardous materials must take steps immediately to cleanse the affected area. Stop working and remove PPE if contaminated. If an aerosol exposure is suspected, leave the area immediately and evacuate the area. Post signs to prevent entry. ISSUE DATE: DD/MONTH/YY Page 11 of 20
12 o Eyes rinse with water for 15 minutes. o Mouth rinse with copious amounts water. o Skin wash the affected area with soap and water. Apply an antiseptic such as alcohol, povidone iodine, chlorhexidine, etc. o Puncture wound allow to bleed freely. Wash the affected area with soap and water. Apply an antiseptic such as alcohol, povidone iodine, chlorhexidine, etc. Once the above first aid is administered, Notify the PI or laboratory supervisor and report to: o Belknap Campus Cardinal Station 215 Central Ave Suite 110 ( ). o HSC Campus Health Care Outpatient Center (HCOC) 401 E Chestnut Suite 110 ( ). o After normal business hours, personnel can be seen at University of Louisville Hospital emergency room located at 530 South Jackson Street for medical evaluation and further treatment/follow-up if necessary. The PI/supervisor is responsible for notifying the BSO of the incident. The employee should complete a Biological Exposure Incident Follow-up Survey (Appendix D of this document.) The supervisor must complete an Employee First Report of Injury form, documenting the route of exposure and the circumstances under which the incident occurred. To access forms go to the Risk Management web-site at Shipment of Potentially Biohazardous Materials All materials known or reasonably expected to contain pathogens must be packaged, labeled, and shipped in accordance with the applicable regulations regarding the transportation of infectious substances. Reasonably expected means that the material has been tested and found to be or contain a pathogen, or that has been taken from a patient known to be infected with a pathogen. Pathogens are defined as microorganisms (including bacteria, viruses, rickettsia, parasites, fungi) and other agents such as prions, which can cause disease in humans or animals. All infectious or potentially infectious materials that are to be air shipped or transported over public highways must also be packaged in accordance with the applicable regulations. NOTE: Anyone involved in the shipment (packaging, labeling, manifesting, etc.) of biohazardous materials or non-infectious materials shipped with dry ice must be trained in hazardous material shipping procedures, and this training must reoccur every 2 years, or more frequently if the regulations change. Training can be found online on ISSUE DATE: DD/MONTH/YY Page 12 of 20
13 the DEHS website. Research personnel who need further assistance in preparing a biological sample shipment can request DEHS assistance by sending the DEHS Shipment Request Form for Biological Samples located on the DEHS website. 6.0 References 29 CFR Part , Occupational Exposure to Bloodborne Pathogens: &p_id=10051 Biosafety in Microbiological and Biomedical Laboratories, CDC-NIH, Fifth edition: WHO Laboratory Biosafety Manual, Third edition: Selection and Use of Disinfectants, U of L Biosafety Manual Working Safely in a Biosafety Cabinet (BSC) for BSL-2, U of L Biosafety Manual 7.0 Appendices Appendix A - DEHS Waste Disposal Guide for Research Labs (BSL-2 and non-bsl) Appendix B - Protection of Vacuum Lines in Laboratories Appendix C - Equipment Decontamination Verification Tag Appendix D - Biological Exposure Incident Follow-up Survey Appendix E - Molded Face Mask Image ISSUE DATE: DD/MONTH/YY Page 13 of 20
14 APPENDIX A DEHS Waste Disposal Guide for Research Labs (BSL-2 and non-bsl) Includes: Contaminated needles, scalpel blades, glass slides, pipette tips, and broken glass Bar Code Label Required: WHITE To obtain bar code labels call Disposal Container: Sharps waste must be placed in a puncture-resistant container with a universal biohazard symbol. Full containers must be securely closed and placed into a red bag lined vendor provided bio-hazardous waste box, red barrel, or gray tote. Only 2 inch wide clear tape can be used to secure boxes. Collected By: U of L Custodial Services HSC Belknap Waste Type: BIOHAZARD BIOLOGICAL WASTE Contents: Infectious microorganisms or OPIM, recombinant DNA, and/or other biohazardous materials. Bar Code Label required: WHITE To obtain bar code labels call Disposal Container: This type of waste is autoclaved/disposed off-site by contracted vendor. Labs must use vendor supplied red bags and bio-hazardous marked, box, tote, or red barrel. Labs must also mark building name, room number, and contact name and phone on each full container. Only 2 inch wide clear tape can be used to secure boxes. Collected By: U of L Custodial Services HSC Belknap Waste Type: PATHOLOGICAL, TRACE CHEMO & TRANSGENIC WASTE Contents: Animal carcasses and parts, human parts, organs and recognizable tissues. Also, trace chemo, inactivated Category A, and transgenic animal and plant waste. Bar Code Label required: YELLOW To obtain bar code labels call Disposal Container: This type of waste is incinerated off-site as medical waste by contracted vendor. No free liquids in red bag; labs should place pads or paper towels to absorb as necessary. Labs must also mark building, room number, and contact name and phone on each full container. Only 2 inch wide clear tape can be used to secure boxes. Collected By: U of L Custodial Services HSC Belknap Waste Type: BIOHAZARD CONTAMINATED PIPETTES AND PIPETTE TIPS Contents: Biohazard contaminated serological pipettes and pipette tips Bar Code Label required: WHITE To obtain bar code labels call Disposal Container: This type of waste is autoclaved/disposed off-site by contracted vendor. To avoid puncture to primary red bag, items should be placed in cardboard box, then placed within red bagged lined vendor provided cardboard box, red barrel, or tote. Collected By: U of L Custodial Services HSC Belknap ISSUE DATE: DD/MONTH/YY Page 14 of 20
15 APPENDIX A (continued) DEHS Waste Disposal Guide for Research Labs (BSL-2 and non-bsl) Waste Type: UNCONTAMINATED PIPETTES AND PIPETTE TIPS Contents: Chemically inactivated by 10% bleach and/or uncontaminated serological pipettes and pipette tips. Decant used bleach solution in sink. Disposal Container: This type of waste is managed as potentially injurious trash. Discard into a sturdy cardboard box lined with non-red colored trash bag. If box is not pre-marked to indicate broken glass, mark box to convey hazard i.e. Caution Broken Glass. Labs must also mark building, room number, and contact name and phone on each full box. Box cannot exceed 20 lbs (9 Kg). Collected by: U of L Custodial Services HSC Belknap Waste Type: GLASS AND PLASTIC LAB WARE, UNCONTAMINATED Contents: Chemically inactivated by 10% bleach and/or uncontaminated intact and broken lab glassware or plastic ware. Decant used bleach solution in sink. Disposal: This type of waste is managed as potentially injurious trash. Discard into a sturdy cardboard box lined with non-red colored trash bag. If box is not pre-marked to indicate Broken Glass, mark box Caution Broken Glass. Labs must also mark building, room number, and contact name and phone on each full box. Box cannot exceed 20 lbs (9 Kg). Collected By: U of L Custodial Services HSC Belknap Waste Type: CHEMICAL WASTE - SOLID, LIQUID, AND COMPRESSED GAS Contents: Includes chemical waste generated by experiments (i.e. solvents, acids, bases, toxic, carcinogens, heavy metals, etc.) and cleaning activities; expired chemicals, reagents and aerosol products. Also, used batteries and lamps (no label required). Disposal: Chemical waste is picked up by DEHS. Attach a uniquely numbered chemical waste label to each container and submit pick up request via on-line form at To obtain numbered labels call Collected By: U of L DEHS Hazardous Waste For more info call Waste Type: RADIOACTIVE WASTE Contents: Materials containing or contaminated by radionuclides. All radioactive materials are marked with the universal radiation symbol. Do not mix with chemical waste. Disposal: All radioactive waste is removed by Radiation Safety. Submit pick up request via on-line form Collected By: U of L DEHS Radiation Safety For more info call Emergency to Life and Health Call 911; Chemical Spills DEHS Disposal Guide ISSUE DATE: DD/MONTH/YY Page 15 of 20
16 APPENDIX B Protection of Vacuum Lines from Biohazardous Material Whenever the house vacuum or a portable vacuum pump is used with potentially biohazardous material, the vacuum line must be protected with a disinfectant trap and filter. The configuration will include, working out from the vacuum nozzle, an in-line filter, a disinfectant trap and a collection flask. If the collected material is to be discarded, the collection flask (A) should contain the required amount of disinfectant. This is sufficient volume to achieve a final concentration (if the vessel were full) of at least 10% bleach in waste. Waste treatment time prior to sewer disposal is a minimum of 30 minutes The overflow disinfectant trap (B) should be an Erlenmeyer flask with a stopper having plastic tubing extending approximately halfway into the disinfectant solution. A 1-3 L Erlenmeyer flask filled approximately 1/10-1/4 full with disinfectant is recommended. Flasks should be plastic, plastic-coated glass or glass covered with safety netting. Acceptable disinfectants for the overflow trap are as follows: A minimum of a 10% solution of bleach in water Wescodyne (in ratio of 1 ml Wescodyne/108 ml of water) The disinfectant solution should be changed at least once a month. If the solution evaporates to below the level of the tubing or becomes contaminated by an overflow of waste, it should be changed at once. The final in-line filter (C) should be a high efficiency particulate air (HEPA) filter or a filter of equivalent or superior efficiency suitable for use in vacuum lines (e.g., Pall VacushieldTM Vent Device) The filter should be changed every six months or sooner if there is any indication of back-up or overflow. If the disinfectant traps are located outside the BSC, they should be sitting in a leak-proof secondary container of some type, such as a plastic dishpan. Drawing: Office of Health and Safety, Centers for Disease Control and Prevention, 1600 Clifton Road N.E., Mail Stop F05 Atlanta, Georgia 30333, USA Last Modified: 1/2/97 ISSUE DATE: DD/MONTH/YY Page 16 of 20
17 APPENDIX C Equipment Decontamination Verification Tag ISSUE DATE: DD/MONTH/YY Page 17 of 20
18 APPENDIX D Biological Exposure Incident Follow-up Survey Survey Questions Prepared by: Date: Notes: Did the incident involve materials containing: Recombinant nucleic acid? Yes No Recombinant Viral Vector(s)? Yes No Infectious Agent(s)? Yes No Genus & Species: Risk Group: RG-1 RG-2 RG-3 At what Biosafety Level is this agent handled BSL-1 BSL-2 BSL-3 ABSL-1 ABSL-2 ABSL-3 BSL-1P BSL-2P BSL-3P Did the incident involve personal injury? Yes No Skin puncture with a needle? Yes No Injury resulting in a break in the skin? Yes No Failure of personal protective equipment (i.e. latex glove breaks and unbroken skin is exposed)? Yes No Splash onto: Unbroken skin? Yes No Broken skin? Yes No Mucous Membranes? Yes No Eyes? Yes No Mouth? Yes No Nose? Yes No Other? Was the injury or potential exposure immediately recognized? Yes No Was appropriate treatment immediately started? Yes No Flush affected area with water? Yes No NA Wash affected area with soap and water? Yes No NA Use of eyewash or safety shower? Yes No NA Other? Was the supervisor immediately notified? Yes No If not, how much time elapsed before notification? ISSUE DATE: DD/MONTH/YY Page 18 of 20
19 APPENDIX D (continued) Biological Exposure Incident Follow-up Survey Was the Principal Investigator immediately notified? Yes No If not, how much time elapsed before notification? Was a health care provider consulted? Yes No By phone? Yes No In person? Yes No Was a follow-up consultation required? Yes No Was the medical professional informed about what you were working with when exposed? Yes No Was the Biosafety Officer informed of the incident? Yes No? Don t know Within 30 days of the incident did you develop any illnesses, symptoms, fever, coughing, etc.? Yes No If yes, do you have any reason to suspect that these are related to the incident? Did you see a health care provider about these symptoms? Yes No Have any changes in laboratory practices been instituted to prevent a recurrence of the incident? Yes No Were other laboratory personnel who also perform this type of work notified of the incident? Yes No Notify worker that pre-existing medical conditions could heighten the seriousness of the exposure and to discuss with healthcare provider if needed. To be completed by PI or supv. Discuss methods for prevention of incident recurrence. ISSUE DATE: DD/MONTH/YY Page 19 of 20
20 APPENDIX E Molded Face Mask Image ISSUE DATE: DD/MONTH/YY Page 20 of 20
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
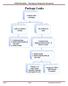 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
What is infection control?
 Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN
 ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
Bloodborne Pathogens Safety in Your Workplace
 Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
BLOODBORNE PATHOGENS
 MARICOPA COUNTY SHERIFF S OFFICE POLICY AND PROCEDURES Subject Related Information CRITICAL POLICY PURPOSE BLOODBORNE PATHOGENS Supersedes CP-6 (08-14-15) Policy Number CP-6 Effective Date 11-22-16 The
MARICOPA COUNTY SHERIFF S OFFICE POLICY AND PROCEDURES Subject Related Information CRITICAL POLICY PURPOSE BLOODBORNE PATHOGENS Supersedes CP-6 (08-14-15) Policy Number CP-6 Effective Date 11-22-16 The
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Spring 2005 Pollution Prevention Workshop For Healthcare
 Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
SKIDMORE COLLEGE. Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS. Introduction -- Pg. 2
 SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard
 ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
Original Date:
 Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL. Table of Contents
 EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan
 Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan (last revised Aug 30, 2011) The Maurice Kanbar Center for Biomedical Engineering Room 704 NAB David Wootton, Ph.D. Director
Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan (last revised Aug 30, 2011) The Maurice Kanbar Center for Biomedical Engineering Room 704 NAB David Wootton, Ph.D. Director
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION
 TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION 1. GENERAL PRACTITIONER INFORMATION New Registration Annual Registration Updated Registration FULL LEGAL NAME (Give
TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION 1. GENERAL PRACTITIONER INFORMATION New Registration Annual Registration Updated Registration FULL LEGAL NAME (Give
Medical Waste Management Plan
 Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control
 The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control Author University Health Service, The University of Hong Kong Approved By Task Force on Infectious Diseases,
The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control Author University Health Service, The University of Hong Kong Approved By Task Force on Infectious Diseases,
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety
 University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety 1.0 Introduction... 1 1.1 Purpose... 1 1.2 Regulatory Background...
University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety 1.0 Introduction... 1 1.1 Purpose... 1 1.2 Regulatory Background...
OHIO UNIVERSITY HAZARD COMMUNICATION PROGRAM (FOR NON-LABORATORY APPLICATIONS) Dept. Name Today s Date Dept. Hazard Communication Contact
 OHIO UNIVERSITY HAZARD COMMUNICATION PROGRAM (FOR NON-LABORATORY APPLICATIONS) Dept. Name Today s Date Dept. Hazard Communication Contact rev. 01/09/07 INDEX SCOPE 3 PURPOSE 3 REFERENCES 3 DEFINITIONS
OHIO UNIVERSITY HAZARD COMMUNICATION PROGRAM (FOR NON-LABORATORY APPLICATIONS) Dept. Name Today s Date Dept. Hazard Communication Contact rev. 01/09/07 INDEX SCOPE 3 PURPOSE 3 REFERENCES 3 DEFINITIONS
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions
 Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
Infectious Waste Contingency Plan
 Infectious Waste Contingency Plan Office of Environmental Health and Safety September 2016 Table of Contents I. Introduction..3 II.Facility Identification and Contact Information..3 III. Emergency Contacts...3-4
Infectious Waste Contingency Plan Office of Environmental Health and Safety September 2016 Table of Contents I. Introduction..3 II.Facility Identification and Contact Information..3 III. Emergency Contacts...3-4
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
Management Plan for Employee Right-to-Know (ERK)
 Management Plan for Employee Right-to-Know (ERK) Table of Contents: Annual Review Form 1.0 Purpose 2.0 Globally Harmonized System (GHS) Compliance 3.0 Identification of Workplace Hazards 4.0 Hazard Assessments
Management Plan for Employee Right-to-Know (ERK) Table of Contents: Annual Review Form 1.0 Purpose 2.0 Globally Harmonized System (GHS) Compliance 3.0 Identification of Workplace Hazards 4.0 Hazard Assessments
