Biohazardous Waste Basics
|
|
|
- Aron Nichols
- 5 years ago
- Views:
Transcription
1 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes any waste item that is contaminated with a biological material that is an infectious disease transmission risk or an environmental release risk (i.e., recombinant DNA). In the state of Tennessee, some forms of biohazardous waste are defined as medical wastes and are regulated for disposal purposes by the Tennessee Department of Environment and Conservation (TDEC). These regulated medical wastes include the following categories which may be applicable to UT researchers: 1. Wastes generated by hospitalized patients who are isolated to protect others from communicable diseases; 2. Cultures and stocks of infectious agents, including specimen cultures from medical and pathological labs, cultures and stocks of infectious agents from research and industrial labs, wastes from the production of biological, discarded live and attenuated vaccines, and culture dishes and devices used to transfer, inoculate and mix cultures; 3. Waste human blood and blood products such as serum, plasma and other blood components; 4. Pathological wastes (i.e., tissues, organs, body parts, and body fluids) that are removed during surgery and autopsy; 5. All discarded sharps (i.e., hypodermic needles, syringes, Pasteur pipettes, broken glass, scalpel blades) used in patient care or which have come into contact with infectious agents during use in medical, research or industrial labs; 6. Contaminated carcasses, body parts, and bedding of animals that were intentionally exposed to pathogens in research in the production of biologicals, or in the in vivo testing of pharmaceuticals. Unlike hazardous chemical or radioactive waste, there is no one federal agency that clearly defines and regulates biohazardous waste. Several agencies associated with research funding have unique waste disposal requirements that may go above and beyond what TDEC regards as regulated medical waste. Therefore, it is the researcher s responsibility to have a general knowledge of biosafety regulations & guidelines and how they apply to their work and the waste that is generated through the research and diagnostic service process. Please review the regulatory/agency information in the following table. If your work will involve generating any of the wastes previously described, or any of the wastes in the table, then you will most likely need to segregate and manage some portion of your research waste as biohazardous waste. Questions about biohazardous waste treatment and disposal? Please contact the Biosafety Office at or Page 1 of 14 UT Biosafety 07/2012
2 Other Agencies with Biohazardous Waste Requirements Regulation Activities covered by this standard Biohazardous wastes OSHA s Bloodborne Pathogens Standard NIH Guidelines for Research Involving Recombinant DNA Molecules CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL) USDA APHIS Permits Work with human-derived materials including clinical and unfixed anatomical specimens, human cells and cell lines. Work with molecules that are constructed outside living cells by joining natural or synthetic DNA segments to DNA molecules that can replicate in a living cell, or molecules that result from the replication of those previously described; regardless of whether work meets exempt criteria or not, all recombinant DNA work is to be carried out in accordance with biosafety level 1 containment practices at a minimum. Lab and animal studies involving work with microorganisms that can cause disease in humans; under certain circumstances, lab and animal studies involving microorganisms that are infectious to animals; diagnostic laboratory operations involving human or animal clinical specimens. Work with any animal or plant-derived materials or pathogens that require an APHIS permit to receive or retain the material. Those wastes that are contaminated to the extent where fluids can drip off or flake off of waste; liquid wastes; fresh (unfixed) tissues; sharps. All contaminated solid and liquid wastes including sharps. All cultures, stocks and items contaminated with these materials; in some cases, animal bedding and carcasses; biohazardous sharps. Permits will outline specific waste treatment requirements for the material in question. However, this usually involves segregation and biological inactivation of the material prior to disposal. Managing Lab Waste as Biohazardous as a Prudent Practice The unique research enterprise at The University of Tennessee covers a wide range of activities including agricultural, environmental, and a wide array of medically-oriented research. Some of these activities may fall outside the regulatory guidelines established by the Federal and State Government. Applying a universal precautions approach (i.e., managing all research biological materials as if they are an infectious disease or environmental release risk) in conducting these varied research activities is a prudent standard. The University of Tennessee Institutional Biosafety Committee and Office of Research recommend that otherwise unregulated research lab biological wastes be managed as biohazardous waste. This action will assure that all biological research materials are inactivated or managed in a manner that isolates the exposure risk for the general public and the environment. Attached Resources Attachment A- Managing Serological Pipettes for Disposal Attachment B- Transporting Biohazardous Waste Attachment C- Autoclave Validation & Biohazardous Waste Treatment Procedure Page 2 of 14 UT Biosafety 07/2012
3 Biohazardous Waste Categories There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate manner for the form in order to minimize occupational exposure and environmental release risks. Biohazardous waste in any form should not be left untreated and unsecured in areas that are accessible to the public (i.e., left in hallways). Only lab personnel should remove treated biohazardous waste from the lab area and transport it to waste holding areas for final disposal. 1. SOLID BIOHAZARDOUS WASTE (non-sharps) In the research lab or field environment, this includes any non-sharp item that is contaminated with human or animal diagnostic specimen material (i.e., body fluids, tissue debris), any microbiological culture material (including recombinant DNA). Examples include but are not limited to: Gloves and other disposable PPE contaminated with specimen or culture material. Plasticware such as pipettes or pipette tips, culture plates, specimen vials, etc. that are contaminated with biological specimens, bacterial and cell culture material, or nucleic acids. Towels and bench paper that are biologically contaminated (Note: Bench paper that is used in areas where samples or cultures are opened and manipulated must be regarded as biologically-contaminated and therefore removed and managed as solid biohazardous waste) All culture or sample containers that are contaminated with biological materials Tubes of blood (note: glass blood vials that could break easily upon disposal should be segregated as sharps waste; see below) Storage This type of waste must be collected for final treatment and disposal in a leak-proof container lined with a bag of moderate thickness to prevent punctures. The collection container must have a lid or other means of closure and the container must be labeled with the biohazard symbol regardless of the lab s operating biosafety level. Bench top containers should be used for collection of small quantities of contaminated dry goods (i.e., pipette tips, centrifuge tubes, etc.). Small plastic containers or wire bag racks lined with a biohazard bag are suitable for bench top collection. These containers do not need to have a lid (unless waste is contaminated with a pathogen) but daily disposal of the bag into a larger collection container such as the one shown to the right is strongly recommended. Wastes Requiring Special Considerations Breakable biohazardous wastes Tubes of blood and other breakable biohazardous waste can be troublesome to manage properly and safely for treatment and disposal. For small amounts of breakable biohazardous waste, these items may be placed in sharps containers for disposal. However, if your lab generates a large amount of breakable biohazardous waste, please contact the UTK/UTIA Biosafety Officer for assistance with finding solutions for safer waste management. Page 3 of 14 UT Biosafety 07/2012
4 Serological pipettes All plastic pipettes, regardless of contamination status should be segregated from other lab wastes because they readily puncture waste and trash bags which increases spill potential. Please see Attachment A at the end of this document for procedures for effective segregation, treatment and disposal of serological pipettes. Treatment and disposal The purpose of solid biohazardous waste treatment is biological inactivation in a manner that reduces hazardous exposure risk for lab personnel and the environment. This is generally achieved by autoclave treatment of waste or treatment and disposal through a medical waste disposal contractor (i.e., licensed medical waste hauler) who will autoclave or incinerate the waste. Under the TDEC regulations, wastes are to be rendered non-infectious by sterilization techniques prior to disposal. This means that all items contaminated with a potentially infectious material must be autoclaved or managed through a medical waste disposal contractor for disposal. Disinfection of items such as serological pipettes contaminated with human cells does not preclude the need to manage these items as biohazardous waste for final treatment and disposal. On-site Autoclave Treatment Autoclavable waste bags must be used in biohazardous waste collection containers. Bags must be placed in a secondary container (i.e., tray with raised sides), which is placed on a cart for movement to the autoclave facilities. Practice notes on biohazard bags: Biohazard bags are a one-way means of disposal. Do not dump the contents from one biohazard bag into another as this action spreads contamination and increases your exposure to this waste. Biohazard bags need to be contained at all times during the collection, treatment, and disposal process. Some lab items may puncture bags and this can lead to leaks and spills. Bags awaiting autoclave treatment should be stored in trays, tubs, buckets, etc. (The only exception to this practice is when small quantities of biohazardous wastes that do not contain liquids are collected temporarily in benchtop containers.) Biohazard bags must not be used for collection of other hazardous wastes (i.e., ethidium bromide gels). Biohazardous waste bags awaiting treatment should always be stored in pans or secondary containment to prevent spills! Ethidium bromide waste is to be collected in a leak-proof container with a lid lined with a sturdy, non-descript bag. The UT Hazardous Waste label should be placed on the storage container as well. No biohazard labels! Page 4 of 14 UT Biosafety 07/2012
5 If your lab works with human-derived materials or other materials that are an infectious disease risk for humans (i.e., BSL-2), you must use bags that bear the biohazard symbol. The bags should have a built-in heat indicator that allows for verification of autoclave treatment. Otherwise, autoclave indicator tape should be placed on the bag before autoclave treatment. If your lab does not work with human-derived materials or other materials that are an infectious disease risk for humans (i.e., BSL-1), you may use bags that do not have the biohazard symbol. However, all biohazardous waste must be clearly identified as such from the point of generation through the point of treatment or biological inactivation. Therefore, if you chose to use autoclave bags without a biohazard symbol, you must employ some other means to identify the material as a biohazard. An example of how this can be achieved is placing the waste in a designated, biohazard-labeled secondary container for storage and transport. The waste bag can then be transferred from the biohazard-labeled secondary container to a regular autoclave pan for final placement in the autoclave. Autoclave treatment of this waste must be performed in accordance with the biohazardous waste treatment parameters established for the autoclave. Note: Only personnel who have received training regarding the operation of the autoclave should use this device. If the treated wastes meet the TDEC definition of medical wastes, or if bags are red/orange in color and/or have a biohazard symbol on them, they must be placed in designated Autoclave-treated Waste bins. These bins are large white waste receptacles, are clearly labeled, and are typically in or near autoclave rooms. This action is required because these treated wastes must be segregated from other solid wastes. The treated wastes are subsequently relocated by Facilities Services to a designated dumpster to be hauled directly to an approved landfill per TDEC requirements. Medical Waste Disposal Contractor Some departments have a contract for pickup and disposal of waste through a medical waste disposal contractor. In this case, the same procedures apply as above. However, bags do not have to be autoclavable, nor should they be placed in a non-see-through black bag. Field Generation Waste should be collected and stored as previously outlined. Contact the UTK/UTIA Biosafety Officer or UTIA Safety Officer for assistance with identifying disposal options. If you plan to transport waste for treatment and disposal, please refer to guidelines at the end of this document for this activity. 2. LIQUID BIOHAZARDOUS WASTE This includes bulk quantities of blood, blood products, body fluids from human and animal research origin and culture media. Note: Disposable primary containers or sample containers containing small quantities of liquids (less than 10 mls) should be managed as solid biohazardous waste. Page 5 of 14 UT Biosafety 07/2012
6 Storage These liquids must be stored in closed, leakproof containers while awaiting treatment and disposal. Collection vessels should be secured so that they can not be tipped over. Secondary containment is strongly recommended and can be achieved by placing the vessel in a bucket or deep tray. Storage vessels or the secondary container must be labeled with the biohazard label if the liquids will not be treated and disposed of within the shift. If disinfectant is added to the vessel, provide labeling so that the chemical hazard is identified as well. For instance, if your collection flask contains waste cell media and bleach, you should place biohazard label on the flask (or secondary container) as well as the words bleach-treated cell culture materials to properly identify both the chemical and biological hazard. Treatment and disposal Liquid wastes may be treated and disposed of by either one or the other of the following methods: Chemical treatment of liquids with disinfectant; disposal via lab sink Disinfectants may be used for treatment of liquid biological waste to prohibit growth of microorganisms. Here is an example for the use of household bleach. Add household bleach to the collection vessel so that the bleach makes 10% to 15% of the final volume. Allow a contact time of at least 30 minutes. Carefully discharge the mixture to the sanitary sewer by way of the lab sink, then thoroughly rinse down the sink with water. Remember to wear splash goggles, gloves, and a lab coat for handling of bleach and bleach-treated liquids. NOTE: Diluted bleach solutions may go down the drain in most cases. However, many chemicals used for disinfection cannot be discarded down the train. Contact EH&S at to determine if sink disposal of disinfectants other than diluted bleach solutions is acceptable. Autoclave treatment of liquids; disposal via lab sink Place the closed collection vessel in a secondary container and transport by cart to the autoclave facilities. Treat by autoclave using the liquids cycle. (Remember to loosen or remove the closure on the vessel before placing in autoclave.) Discharge cooled, treated liquids to the sanitary sewer by way of the lab sink. Note: Only personnel who have received training regarding the operation of the autoclave should use this device. Safety Note: PLEASE do not autoclave liquids containing chemical disinfectants! 3. BIOHAZARDOUS SHARPS A biohazardous sharp is any device that is sharp enough to puncture the skin and that is contaminated with a biological material that is an infectious disease transmission risk, or an environmental release risk (i.e., recombinant DNA). Examples include but are not limited to: Needles, disposable syringes, capillary tubes & scalpels contaminated with human or animal blood Microscope slides contaminated with unfixed human or animal specimen materials Pasteur pipettes contaminated with cell culture waste media Small glass/broken tubes of blood or microbiological cultures. Page 6 of 14 UT Biosafety 07/2012
7 SAFETY NOTE ON NEEDLES & MEDICAL DEVICES WITH A NEEDLE ATTACHED State waste regulations do not specifically address the disposal of hypodermic needles and medical devices with a needle attached (i.e., tuberculin syringes) unless these items are contaminated with blood or potentially infectious materials. However, it is strongly recommended that ALL needles and medical devices with a needle attached be disposed of in biohazardous sharps containers to protect those who may come in contact with these items during the disposal process. If such devices are contaminated with radioactive materials- follow Radiation Safety procedures for disposal of such items. If such devices are contaminated with hazardous chemicals, contact the Environmental Health and Safety (EH&S) Office at , or the UTIA Safety Officer at for guidance with disposal. Storage Biohazardous sharps containers are those containers which are specifically designed for the collection and disposal of biohazardous or medical sharps. (Recycled food or reagent containers are NOT acceptable for collection and disposal of biohazardous sharps!) A biohazardous sharps container is: constructed of puncture-resistant material, leak-proof on the sides and bottom, marked with the biohazard symbol, and has a restricted opening to prevent items from coming back out of the container, and to prevent someone from sticking their hand inside. To protect yourself and others in your work area, place biohazardous sharps in a properly assembled (i.e., lid installed) biohazard sharps container immediately after use. This can be achieved by placing sharps containers within arms reach of where biohazardous sharps are used. SAFETY NOTE: Do not recap needles. Do not bend or break sharp devices. Do not overfill sharps containers or use force to get an item into a sharps container. Treatment and disposal All sharps containers must be permanently closed and disposed of when ¾ full or whenever items do not freely fall into the container. Disposal of biohazardous sharps containers will be accomplished through a medical waste disposal contractor coordinated through EH&S. Please do not dispose of biohazardous sharps containers in the trash, regardless of treatment status. Collection will be on the same schedule as chemical waste. (Knoxville campus on Wednesdays at Walters waste room from 1-2 pm and SERF waste room from 2-3 pm. Ag Campus on quarterly waste pickup days and by appointment by calling the UTK/UTIA Biosafety Officer at ) Sharps containers must be permanently closed and wiped down with a disinfectant prior to removal from the lab and for disposal through EH&S. If there are any liquids present in the biohazardous sharps container, it must be placed in a leak-proof secondary container with a secure lid (and a biohazard label) for transport to the waste collection site. Upon arrival at the waste room, the container must be placed in the large, cardboard waste container by the generator. Page 7 of 14 UT Biosafety 07/2012
8 Medical Waste Disposal Contractor The same procedures apply as above. Do you have long Pasteur pipettes that are contaminated with potentially infectious material or recombinant DNA? Field Generation Waste should be collected and stored as previously outlined. Contact the UTK/UTIA Biosafety Officer or UTIA Safety Officer for assistance with identifying disposal options. If you plan to transport waste for treatment and disposal, please refer to guidelines at the end of this document for this activity. Tall sharps containers with an opening large enough to safely deposit these items are strongly recommended. SAFETY NOTE ON BROKEN GLASS If broken glass is biologically-contaminated, it must be managed as a biohazardous sharp for disposal. However, some instances occur when the broken glass does not fit in a sharps container. In these events, please call the UTK/UTIA Biosafety Officer for assistance. Broken glass that is not contaminated with a hazardous material should be placed in a suitable puncture-resistant container for disposal. (Storage of broken glass in trash bags is NOT acceptable!) Disposable and reusable broken glass containers are available through most lab supply companies. Only lab personnel should remove broken lab glass from the lab area and transport it to waste holding areas for disposal. 4. PATHOLOGICAL WASTE This includes all unfixed human organs, tissues and body parts except for teeth. It also includes unfixed animal tissues and carcasses that have been: exposed to human-derived materials (i.e., cells), experimentally challenged with agents infectious to humans or recombinant organisms, and other circumstances as deemed appropriate through the biological risk assessment process. Page 8 of 14 UT Biosafety 07/2012
9 Storage, treatment and disposal This type of waste must be double-bagged in biohazard bags that bear a biohazard symbol. Bags must be stored in a manner that will minimize the potential for release of fluids during the storage and handling process. Storage of bags in a tray with sides, or secondary storage of bags in a sturdy plastic zipper bag is strongly recommended. Remember that these items must be labeled with the biohazard symbol. These items must be incinerated (not autoclaved) for disposal unless other provisions apply. A medical waste disposal contractor should be used for pickup and disposal of these materials. Contact the UTK/UTIA Biosafety Officer ( ) for further assistance with disposal of items in this category. For assistance with disposal of formalin-fixed or chemically preserved tissues, please contact EH&S or the UTIA Safety Officer. Final Note on Mixed Wastes Some lab analyses may involve treatment or exposure of biological materials to chemical compounds or radioactive materials. Examples may include radioisotope labeling of genetic material in culture or cells, and exposure of cells or research animals to carcinogens or diagnostic processes involving radiation hazards. In these situations, mixed wastes are likely to be produced that will require special consideration for collection, handling and disposal. Biohazardous waste treatment and disposal techniques alone are not likely be suitable for mixed wastes. When planning studies that will generate mixed wastes, please contact the appropriate safety office for assistance in determining your waste handling procedures. Environmental Health and Safety Office UTIA Safety Office Radiation Safety Office UTK/UTIA Biosafety Office Page 9 of 14 UT Biosafety 07/2012
10 Attachment A Recommended Practices: Disposal of Plastic Serological Pipettes All plastic pipettes, regardless of contamination status should be segregated from other lab wastes because they readily puncture waste and trash bags which increases spill potential. Disposal recommendations: Pipettes that ARE NOT biologically-contaminated The following recommendation applies to waste pipettes that ARE NOT contaminated with body fluids, cell debris, or other materials that may contain infectious agents or recombinant DNA molecules. Pipettes should be placed in a dedicated container that is lined with a sturdy trash bag, and configured in such a way that pipettes are oriented in one direction. The container should be clearly marked waste pipettes only- NO biohazardous waste or comparable wording to ensure that all personnel are notified of the intent of the container. To dispose of the pipettes, the trash bag should be tied closed and transferred to a cardboard box (if it s not stored in one already). The box should be taped closed and trash should be written on the box. It is highly recommended that lab personnel remove the box directly to a nearby dumpster. In some buildings, custodial personnel will remove the box from the lab. Disposal recommendations: Pipettes that ARE biologically contaminated Waste pipettes that ARE contaminated with body fluids, cell debris, or other materials that may contain infectious agents or recombinant DNA molecules must be segregated, stored, treated and disposed of as biohazardous waste. There are various ways that this can be achieved as long as the basic principles of biohazardous waste handling and disposal are followed. Here are examples of acceptable practices: Cardboard boxes are convenient collection containers but they are not leak proof and cannot be disinfected. Therefore, if you use a cardboard box as your collection container, you must use it as a onetime collection container only. Additionally, you should restrict the use of cardboard boxes to circumstances where they will be filled and replaced frequently. Pipette washers or 5-gallon buckets may be lined with a biohazard bag and used for pipette segregation. In this scenario, please ensure that the outside of the container has a biohazard label. When the bag is full, pipettes can be treated by autoclave or disposal through the medical waste hauler. o o If treating by autoclave: After autoclave treatment, bags of pipettes should then be placed in a designated Autoclave-treated Waste containers. If disposing through the medical waste contractor: Transfer the bag of pipettes to a contractorapproved container and close container per provided instructions. Waste pipettes may also be collected in a receptacle containing disinfectant (i.e., pipette washer) at the time of use. A biohazard label and identification of the disinfectant should be on the receptacle. The pipettes should be placed in the receptacle so that the contaminated tips are submerged in the disinfectant. At the conclusion of procedures, the pipettes can be drained and transferred from the receptacle to a biohazard bag for treatment by autoclave, or disposal through the medical waste hauler. o o If treating by autoclave: After autoclave treatment, bags of pipettes should then be placed in a cardboard box. The box must then be securely taped closed and clearly marked as trash. If disposing through the medical waste hauler: Transfer the bag of pipettes to a cardboard box. The entire box must be closed and placed in a biohazard bag for treatment and disposal through the medical waste hauler. Please contact the Biosafety Officer at if you are using a different system to manage your pipettes to determine if it meets the biohazardous waste handling requirements. Page 10 of 14 UT Biosafety 07/2012
11 Attachment B Procedure for Transporting Biohazardous Wastes Whenever possible, biohazardous wastes should be treated and disposed of on-site. However, generation of biohazardous wastes in the field is often unavoidable and handling and transport will be necessary. Please follow the steps below in order to manage and dispose of these materials safely. 1. Wastes that are generated in the field must be segregated and collected using the same principles as outlined for the lab environment. 2. Bags of waste and sharps containers should be closed before removal from the site. (Bulk quantities of liquid waste should not be transported if at all possible. Contact the UTIA/UTK Biosafety Officer for assistance if the need arises to transport such material.) 3. Bags of waste and sharps containers must be placed in a leak-proof secondary container with a secure lid (i.e., latchable, secured with tape, etc.) for transport to treatment facilities. The secondary container must be labeled with a biohazard symbol and an emergency contact name and phone number. 4. Use a University-owned vehicle whenever possible for transport. Store and secure the transport container in a location in the vehicle whereby if an accident were to occur, the container or its contents will not be an exposure risk to the driver or the environment. For example, if transporting materials by car or van, store the container in the back seat or cargo bay. Secure the container with bungee cords or belts to keep the container upright and stable. 5. When you arrive at your destination, transport the waste into the facility using the shortest available route, and move the materials with the aid of a cart. Do not use public elevators if at all possible and avoid traveling with the waste through common public areas. Do not touch door handles, elevator buttons or other common contact surfaces with gloved hands. (Use the one-gloved hand technique, or get assistance from other staff for opening doors, etc.) Page 11 of 14 UT Biosafety 07/2012
12 Attachment C Autoclave Validation for Biohazardous Waste Treatment In accordance with local and state regulations, all biohazardous waste must be biologicallyinactivated before it is disposed of as regular trash. This can only be achieved if the waste is exposed to the right temperature for the right amount of time. Optimally, the waste should be exposed to: 121 degrees C, at a pressure of 15 PSIG for at least 20 minutes. Autoclave gauges are not always accurate, and autoclave tape only indicates the presence of hot steam. Therefore, the autoclave must be validated on a regular basis with a biological indicator ampoule or chemical integrator strip that clearly demonstrates that the appropriate conditions were achieved for sterilization. Responsibility for validating autoclave performance lies with those who use the autoclave for treating biohazardous waste. It is strongly recommended that a designated individual be identified among the lab staff who will be responsible for the validation of the autoclave and the training of personnel who use this equipment. UT Biosafety Recommended Practice for Autoclave Validation and Use Each quarter all autoclaves used to treat biohazardous waste will be validated using 3M Comply Thermalog Steam Chemical Integrator strips, which will be provided by the Biosafety Office upon request ( or ). Chemical integrator strips test the time, temperature, and quality of steam exposure and are calibrated to mimic the results obtained using the traditional Bacillus stearothermophilus biological indicator tests (i.e., spore tests). However, chemical integrator strips give immediate results rather than requiring the hour postautoclave incubation period necessary for spore germination and growth. Based on prior parallel validation testing experiments, there is no difference between the chemical integrators and biological indicators. However, the Biosafety Office will perform spore tests along with the chemical integrators if validation problems/inconsistencies arise. One misconception that often arises is that biological waste is inactivated as long as the autoclave chamber achieves the proper conditions. However, remember that autoclave performance is affected by load size (volume) and load contents. For example, 121 degrees C for 15 minutes may be adequate to sterilize 2 L of media but not 20 L of media. Thus, achieving proper chamber conditions does not always guarantee sterilization for the entire load. This is especially true for bagged biohazardous waste. Complete inactivation of bagged biowaste can be a challenge and often requires a few additional procedural precautions. The table below illustrates two common problems likely to negatively impact the complete inactivation of bagged biowaste and tips to remedy these problems. Problems Inadequate steam penetration and exposure Overstuffing the chamber Solutions Biohazard bags are not usually steam permissive, so: 1. Be sure that bags are open. Do not close the bags by binding or tying prior to autoclaving. 2. Add a small amount of warm water to the bag to generate steam within the bag. 3. Increase the sterilization time. Bagged biowaste is often bulky or high volume, so: 1. Do not overfill biohazard bags. 2. Be sure that the autoclave fits the task. Do not cram large bags into autoclaves that are too small to accommodate the load. 3. Do not autoclave multiple biohazard bags. Page 12 of 14 UT Biosafety 07/2012
13 To summarize the details specified above, the Biosafety Office has developed a standard protocol for the validation of autoclaves used to treat biohazardous waste. The autoclave validation procedure is outlined below: Autoclave Validation Instructions 1. Place a full, medium-sized biohazard bag (e.g. 25" x 35") into an autoclavable secondary container. 2. Add 1 cup (~250ml) warm water to the bag. This is recommended for all bagged waste, but it is required if the contents are not likely to generate steam within the bag (e.g. bag contains mostly dry plastic disposables). 3. Place a 3M Comply Thermalog Steam Chemical Integrator strip inside the bag near the center of the bagged contents. Make sure that there is a mechanism for retrieving the strip after the validation test. For example, affix a piece of twine to the strip and tape one end of the twine to the outside of the bag. 4. Place bag into the autoclave, leaving the top of the bag open to facilitate adequate steam penetration into the bag. Make sure that the bag is opened widely at the top and that it is not bound, tied, twisted. Also, take care not to obstruct the opening of the bag by cramming the bag against the walls or ceiling of the autoclave chamber. 5. Autoclave the biohazard bag for a minimum of 30 minutes at 250F/121C. 30 minutes is the recommended minimum, but sterilizations of >1 hour are not abnormal depending on the autoclave and load volume/contents. 6. After cycle completion, note the status of the chemical integrator. A successful test is achieved only if the blue indicator line reaches any portion of the Safe window. 7. Document the validation test. Documentation should include the following: Date and time of test Load contents Parameter setting for autoclave Type of test and results of test Name of person performing test Failed attempts and remedial actions as necessary 8. If the validation was successful the biohazard bag may be tied up and discarded into a non-see-through trash bag for final disposal. If the validation was not successful, repeat the test and add 10 minutes to the sterilization cycle. Integrator strips are single-use only. Use a new strip for each retest. The autoclave settings that are validated as necessary to achieve effective sterilization of wastes should be communicated to the Biosafety Office so that a database of autoclave validation results can be maintained. Additionally, the Biosafety Office will generate a sign indicating the validated cycle and time settings to be printed and posted by the autoclave tester. Autoclaves that are not validated for biohazardous waste will also be posted accordingly. Examples of autoclave postings are provided on the following page: Page 13 of 14 UT Biosafety 07/2012
14 This autoclave is currently approved for BIOHAZARDOUS WASTE TREATMENT using the following parameters: Cycle Setting Liquids 6 Cycle Time 55 minutes* * This is the validated time for ONE bag of waste. You must add 10 minutes cycle time for each additional bag. REMEMBER: Bags must be in autoclavable secondary container. Bags must be left OPEN for treatment. One cup of WARM WATER must be added to each bag. This validation expires: 6/10/2007 Validation performed by: Cathy Scott Questions? Call or THIS AUTOCLAVE IS NOT CURRENTLY VALIDATED FOR BAGGED BIOHAZARDOUS WASTE! Please contact the Biosafety Office at or for more information on this autoclave or for autoclave validation instructions. Because autoclaves present a number of hazards, only those personnel who have received onsite training by the lab s designated trainer should operate an autoclave. A general procedure for autoclave treatment of solid biohazardous waste (non-sharps) is as follows: Biohazardous Waste Treatment by Autoclave 1. Use secondary containment (i.e. cart) for transporting waste bags to the autoclave for treatment to reduce the possibility of a spill during transport. 2. Add one cup of water to each bag to facilitate air displacement and enhance steam generation. 3. Leave bags open to facilitate steam penetration. 4. Place bags in autoclavable secondary containment pan for autoclave treatment to reduce the possibility of a spill during treatment. 5. Follow waste cycle parameters established for the autoclave to assure effective decontamination of waste. 6. Unload waste after cycle is complete and chamber pressure has returned to 0 PSIG. Do NOT override safety features to open the autoclave. 7. Use autoclave gloves and appropriate eye protection to avoid injury from contact with hot surfaces or liquids when removing waste from autoclave. 8. Tie or band the treated bags closed to reduce the possibility of a spill. 9. Package waste in non-see-through bag or container for final disposal as regular trash. Do NOT autoclave wastes that are contaminated with hazardous chemicals! Page 14 of 14 UT Biosafety 07/2012
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Medical Waste Management Plan
 Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Spring 2005 Pollution Prevention Workshop For Healthcare
 Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
MEDICAL WASTE MANAGEMENT PLAN
 MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Medical Waste Manual. California State University, Chico
 California State University, Chico The Department of Environmental Health and Safety Revised January 4, 2011 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal
California State University, Chico The Department of Environmental Health and Safety Revised January 4, 2011 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Medical Waste Manual. California State University, Chico
 California State University, Chico The Department of Environmental Health and Safety March 2017 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal of Biohazardous
California State University, Chico The Department of Environmental Health and Safety March 2017 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal of Biohazardous
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
UPEI Waste Disposal Protocol
 UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Infectious Waste Contingency Plan
 Infectious Waste Contingency Plan Office of Environmental Health and Safety September 2016 Table of Contents I. Introduction..3 II.Facility Identification and Contact Information..3 III. Emergency Contacts...3-4
Infectious Waste Contingency Plan Office of Environmental Health and Safety September 2016 Table of Contents I. Introduction..3 II.Facility Identification and Contact Information..3 III. Emergency Contacts...3-4
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
KERN HEALTH SYSTEMS POLICIES AND PROCEDURES 2.21-P
 Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
University of Nevada, Reno Operational Plan for Management of Biohazardous Waste
 University of Nevada, Reno Operational Plan for Management of Biohazardous Waste Scope This operational plan covers management of biohazardous waste produced as a result of University of Nevada, Reno (UNR)
University of Nevada, Reno Operational Plan for Management of Biohazardous Waste Scope This operational plan covers management of biohazardous waste produced as a result of University of Nevada, Reno (UNR)
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions
 Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
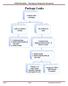 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Safe Handling and Disposal of Sharps. Reference Guide
 Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
UNIVERSITY OF CALIFORNIA SANTA BARBARA Medical Waste Management Plan Large Quantity Generator with Onsite Treatment 417
 UNIVERSITY OF CALIFORNIA SANTA BARBARA Large Quantity Generator with Onsite Treatment 417 Contents I. Introduction II. Definitions III. Responsibilities IV. Types and Quantity of Medical Waste Generated
UNIVERSITY OF CALIFORNIA SANTA BARBARA Large Quantity Generator with Onsite Treatment 417 Contents I. Introduction II. Definitions III. Responsibilities IV. Types and Quantity of Medical Waste Generated
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
BODY ART ESTABLISHMENT PLANNING APPLICATION
 BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL. Table of Contents
 EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
OHIO UNIVERSITY HAZARD COMMUNICATION PROGRAM (FOR NON-LABORATORY APPLICATIONS) Dept. Name Today s Date Dept. Hazard Communication Contact
 OHIO UNIVERSITY HAZARD COMMUNICATION PROGRAM (FOR NON-LABORATORY APPLICATIONS) Dept. Name Today s Date Dept. Hazard Communication Contact rev. 01/09/07 INDEX SCOPE 3 PURPOSE 3 REFERENCES 3 DEFINITIONS
OHIO UNIVERSITY HAZARD COMMUNICATION PROGRAM (FOR NON-LABORATORY APPLICATIONS) Dept. Name Today s Date Dept. Hazard Communication Contact rev. 01/09/07 INDEX SCOPE 3 PURPOSE 3 REFERENCES 3 DEFINITIONS
BODY ART FACILITY CONSTRUCTION PLAN CHECK
 BODY ART FACILITY CONSTRUCTION PLAN CHECK Type of Facility: (mark one) Permanent Temporary/Special Event Are you a: (mark one) New Facility Existing with new ownership Existing Facility Existing remodel
BODY ART FACILITY CONSTRUCTION PLAN CHECK Type of Facility: (mark one) Permanent Temporary/Special Event Are you a: (mark one) New Facility Existing with new ownership Existing Facility Existing remodel
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS
 PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
INFECTION PREVENTION AND CONTROL SAFE USE AND DISPOSAL OF SHARPS
 INFECTION PREVENTION AND CONTROL SAFE USE AND DISPOSAL OF SHARPS Policy title Safe Use and Disposal of Sharps Infection Control and Prevention (IPC) Policy CL05C reference Policy category Clinical Relevant
INFECTION PREVENTION AND CONTROL SAFE USE AND DISPOSAL OF SHARPS Policy title Safe Use and Disposal of Sharps Infection Control and Prevention (IPC) Policy CL05C reference Policy category Clinical Relevant
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Permanent Body Art Facility Plan Review Application
 Permanent Body Art Facility Plan Review Application Livingston County Health Department 2300 East Grand River Suite 102, Howell, MI 48843 Ph:517-546-9858 Fx:517-546-9853 www.lchd.org Authority - Michigan
Permanent Body Art Facility Plan Review Application Livingston County Health Department 2300 East Grand River Suite 102, Howell, MI 48843 Ph:517-546-9858 Fx:517-546-9853 www.lchd.org Authority - Michigan
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
ISO Sharps injury protection Requirements and test methods Sharps containers
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 23907 First edition 2012-09-01 Sharps injury protection Requirements and test methods Sharps containers Protection contre les blessures par perforants
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 23907 First edition 2012-09-01 Sharps injury protection Requirements and test methods Sharps containers Protection contre les blessures par perforants
Infection Prevention Guidelines. Safe Use, Handling & Disposal of Sharps
 Infection Prevention Guidelines Safe Use, Handling & Disposal of Sharps Author: Anne Tateson Owner: Infection Prevention Team Publisher: Infection Prevention Date of Version Issue: February 2015 Version:
Infection Prevention Guidelines Safe Use, Handling & Disposal of Sharps Author: Anne Tateson Owner: Infection Prevention Team Publisher: Infection Prevention Date of Version Issue: February 2015 Version:
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES
 BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
BODY ART ESTABLISHMENT INTRODUCTION GUIDE
 BODY ART ESTABLISHMENT INTRODUCTION GUIDE Toledo-Lucas County Health Department 635 N. Erie Street Toledo, OH 43604 Phone: (419) 213-4100 ext. 3 Fax: (419) 213-4141 INTRODUCTION This guide has been developed
BODY ART ESTABLISHMENT INTRODUCTION GUIDE Toledo-Lucas County Health Department 635 N. Erie Street Toledo, OH 43604 Phone: (419) 213-4100 ext. 3 Fax: (419) 213-4141 INTRODUCTION This guide has been developed
Student Performance Guide. Student Performance Guide. Student Performance Guide. Student Performance Guide. LESSON 3-3 Bleeding Time
 LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
