VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
|
|
|
- Antony Ramsey
- 5 years ago
- Views:
Transcription
1 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive blood culture. 2. Door of BACTEC has pink suspect Ebola sticker. Open to see which bottle is positive. Positive blood culture bottle observed to have pink Ebola sticker. DO NOT REMOVE BOTTLES. Shut door. 3. Notify Supervisor, medical microbiologist on call, and technologist in AFB room. Discuss patient s current status with Medical microbiologist before proceeding. If patient s status is still pending or if they have tested positive for Ebola proceed as described. 4. The TB tech will process the positive blood cultures. 5. The microbiology supervisor will designate a second lab tech to assist. 6. If a blood culture becomes positive in the evenings, technologists will need to be called in. Consult with medical microbiologist. An on call list is provided on the huddle board. 7. Full PPE will be worn by micro technologist when processing positive blood culture. Follow the donning and doffing protocol check list. 8. The processing of the positive blood culture is to be performed by one tech while the other assists by reading and recording the process. AFB technologist: 1. Suspend processing TB samples at an appropriate point. 2. Remove all biohazard waste from the AFB Room. Tie off all bags, label with autoclave before disposal sticker and place in wash up area for autoclaving. It is imperative that all biohazard waste is removed before proceeding. 3. Remove all items from inside the BSC 4. Clear and de-clutter work area outside the hood 5. Begin to prepare the AFB room.
2 Page 2 of 13 A: AFB Room Check list: 1. Turn on the BSC if not already running. Note time and ensure it runs at least 30 minutes before use. 2. Prepare a working area inside the hood by placing down two blue absorbent pads. 3. Place the 95 degree heating block in the BSC, turn on checking temperature setting, and making sure no plastics or flammables are close by. 4. Ensure all the required supplies are ready for use in Ebola positive blood culture designated box following the check list provided. Place on the bench to the right of the hood. 5. Clear the top of the cart to be used for the transfer on the blood culture bottles to the BSC from the BACTEC Instrument. 6. Place inside the hood Oxivir Tb Wipes (ready to be dispensed), Accel TB, and Alcohol based hand rub (1 st set). 7. Prepare Coplin jar with enough methanol to cover the entire surface of a slide and place in BSC. 8. Place all required supplies in the BSC: media, anaerobic jar/indicator (for B bottles only), pack plastic loops, venting units, small red sharps container, frosted slides, slide case, pencil, sharpie, biohazard zip lock bags and double bagged waxed paper waste bucket 9. Ensure 2 copies of doffing protocol (one for each tech) are brought into the AFB room 10. Ensure proper airflow by maintaining adequate space between all objects inside the cabinet and keeping the front grill clear. Refer to instructions for the proper use of BSC (laminated copy is posted near AFB BSC). 11. Place on top of the cart Oxivir Tb Wipes (ready to be dispensed), TB Accel, alcohol based hand rub (2nd set) and a hard plastic TDG container (with absorbent material at the bottom) and lid. 12. Place blue drum and lid for Ebola waste inside AFB room along with all the required drum labels. The blue drum is stored in the wash up room Ensure that the absorbent material provided with the drum is placed at the bottom of the drum and that the drum is double lined with the red biohazard bags provided. 13. Place sign on the door Ebola Containment/Processing DO NOT ENTER.
3 Page 3 of 13 B: Donning of PPE: 1. Follow the lab protocol for donning using the PPE checklist 2. All personnel must be trained in PPE protocol and be fit tested for N95 within the last year. 3. Designated donning area is outside Room Donning will not commence until both technologists are present. 5. Both technologists must sign the Infection Prevention List found in the TDG Area. 6. Both technologists are to be engaged and assist one another, one donning at a time while the other instructs, observes and records the process. C: Subculture of Positive Bactec Bottles: Note: Oxivir Tb Wipes require one minute contact time 1. Wearing full PPE, take the cart carrying the TDG container to the BACTEC Instrument, remove positive blood culture(s) from BACTEC and place in TDG container. Close lid on the TDG container. 2. Using the cart, take TDG container into AFB room, close door and place in BSC. 3. Remove positive blood culture bottles and wipe top of bottles with Oxivir Tb Wipe. 4. Clean outer gloves using Oxivir Tb Wipe or alcohol based hand rub (ABHR). Allow to dry. 5. Label slides and all plates with specimen number and patient name. 6. Vent bottles with venting unit. DO NOT RECAP AFTER USE. 7. Inoculate BAP, MRSS, Chocolate, MacConkey, CNA and, if a B bottle, a Brucella plate. 8. Make smear. Allow to air dry. 9. Using a disposable plastic loop, gently spread out inoculums for isolation being careful not to splash. 10. Clean outer gloves using Oxivir Tb Wipe or alcohol based hand rub (ABHR). 11. As soon as slide is dry, put slide in Coplin jar with methanol making sure entire slide is covered. Set timer for 30 minutes.
4 Page 4 of Clean outer gloves using Oxivir Tb Wipe or alcohol based hand rub (ABHR). 13. Wipe outer surfaces of all plates with Oxivir Tb Wipe allow one minute contact time. 14. Seal all plates with Parafilm, add pink Ebola sticker to each plate, place aerobic plates into a biohazard bag. Wipe surface of Biohazard bag with Oxivir Tb Wipe and label with an Ebola sticker. 15. Place plates into a rack. Apply an Ebola sticker to the rack so that it is clearly visible. 16. Place the anaerobic plates into an anaerobic jar and set the jar up. 17. Wipe the surface of the jar with Oxivir Tb Wipe. 18. Clean outer gloves using Oxivir Tb Wipe or alcohol based hand rub (ABHR). Allow to dry. 19. Incubate primary plates and anaerobic jar in the designated area in the CO2 incubator in AFB room. 20. Carefully remove venting unit from blood culture bottle and place in sharps container. 21. Wipe top of bottles with Oxivir Tb Wipe. 22. Clean outer gloves using Oxivir Tb Wipe or alcohol based hand rub (ABHR). Allow to dry. 23. Place blood culture bottles into biohazard bags and place in new TDG container. Seal container and wipe outer surface with Oxivir Tb Wipe. 24. Label top and sides of TDG container with the accession number and Ebola stickers and store in the BSC until blood culture is finalized. 25. After 30 minutes in methanol, remove slide and allow to air dry. 26. Ensure that the heating block has come to temperature and place slide on 95ºC heating block to inactivate the virus. Set timer for one hour. 27. Wipe the surface of the Oxivir container with an Oxivir Tb Wipe 28. Commence doffing of PPE.
5 Page 5 of 13 D: Doffing of Personnel Protective Equipment The procedure for removing protective equipment is critical and strict adherence to the order of removal of PPE must be followed. Follow VGH Lab Doffing Protocol using the PPE checklist Do not rush! The AFB room is the designated doffing area Both technologists are to be engaged and assist one another in the doffing process, each taking turns doffing while the other instructs, observes, assists and records the process ensuring each step is followed 1. All PPE should be removed in the AFB room and is to be discarded into the blue drum double-lined with red biohazard bags (do NOT compress). 2. Clean hands using ABHR, allow to dry. 3. Leave AFB Room, close door, secure room with sign Suspect Ebola Sample Containment / Processing DO NOT ENTER on door and place barrier (Masking) tape over door to prevent accidental entry. 4. AFB room cannot be used for routine purposes until room cleaning is completed and all Ebola waste is removed. E: Inactivated Blood Culture Slide: Inactivated 1. Donning full PPE, the TB tech will prepare to independently re-enter the AFB room after the slide inactivation is complete (one hour at 95 degrees) Follow the Donning PPE protocol and have an observer to assist. 2. Enter AFB Room. Unplug heating block. 3. Place inactivated slide into slide case and label case with inactivated sticker. 4. Give slide to the blood culture tech (or evening tech) for staining. Blood culture tech will notify medical microbiologist on call to review gram stain and will call the results to the appropriate location. Medical microbiologist on call will discuss with physician per clinical judgement.
6 Page 6 of TB tech will re-enter AFB room to perform room clean up. F: AFB Room Interim Cleanup: Note: Oxivir Tb Wipes require one minute contact time 1. Wearing full PPE, place all disposable waste, into the blue drum that is double lined with red bags. Do NOT press down to compress. 2. Clean gloves with Oxivir Tb Wipe or Alcohol rub. Allow to dry. 3. Proceed with a Primary Clean: Wipe all surfaces inside the BSC and all surfaces used outside the BSC using Accel TB, including the surfaces of containers of any used disinfectants. Using a timer, allow to remain wet 5 minutes and reapply if necessary. Note: The heating block is to remain unplugged inside the BSC until the pm day one work up of blood culture is complete. A clean of the heating block will be done once it has cooled, during the final room clean up. 4. Perform Secondary Clean by repeating the above cleaning process. 5. Clean gloves with Oxivir Tb Wipe or Alcohol rub. Allow to dry. 6. Wipe the surface of the Oxivir Tb container with a Oxivir Tb wipe. 7. When the primary and secondary cleaning is completed, doff PPE in the AFB Room. Follow the doffing protocol and have an observer to assist. Place all PPE into the double-lined blue drum. 8. Exit room and place sign on the AFB room door Ebola processing / containment DO NOT ENTER. Place barrier (Masking) tape over the door to prevent accidental entry.
7 Page 7 of 13 G: PM Growth and Day 1 / Subculture of Primary Plates to Secondary: If patient s status is pending, check with medical microbiologist for any updates before proceeding Ask the Medical Microbiologist to sign a Waste Release Form 1 Prepare supply bin with all required materials and media plates for subculture of growth to secondary plates. Consider the gram stain results and allow for the possibility of more than one colony type being present. Include disposable loops, blue absorbent work surface mats, a double-lined cardboard bucket for waste, a copy of the doffing protocol and 2 routine lab gowns. Take supplies to AFB Room. 2. AFB (or designated) technologist will perform the work up independently but will have an assistant for the donning and doffing following the lab PPE check list. 3. Sign the Infection Prevention Sheet 4. Don PPE with a donning assistant 5. Perform primary and secondary clean of previously used heating block using Oxivir Tb Wipe and allow to remain wet one minute each time. Remove from BSC. 6. Clean gloves with Oxivir Tb Wipes or alcohol-based hand rub (ABHR). Allow to dry. 7. Place blue absorbent pads for a work surface in the BSC. 8. Arrange all materials for use inside hood including Oxivir Tb Wipes and alcohol base hand rub (ABHR). Ensure proper air flow according to safe practice use of a BSC (see instructions for proper use of BSC posted near BSC) 9. Remove primary plates from incubator and place into BSC. 10. Remove plates from biohazard bag and wipe outside of plates with Oxivir Tb wipes. 11. Read plates; observe growth colony characteristics, consistent with Gram stain? 12. If there is no growth, re-wipe plates with Oxivir Tb Wipe, replace Parafilm and place back in biohazard bag. Re-incubate.
8 Page 8 of Clean gloves with Oxivir Tb Wipes or Alcohol based hand rub (ABHR). Allow to dry. 14. Subculture growth to secondary plates. Pick the top of colony in the last quadrant with growth. 15. Do multiple subcultures if different colony types are present. Assistant may record numbered workups if required. 16. Clean gloves with Oxivir Tb Wipes or alcohol-based hand rub (ABHR). Allow to dry. 17. Wipe outside of entire surfaces of secondary plates with Oxivir Tb Wipe. 18. Seal all plates with Parafilm, place an inactivated label on each plate, place in biohazard bag, add inactivated sticker to bag. Wipe outside of biohazard bag with Oxivir Tb Wipes and allow to dry. 19. Clean gloves with Oxivir Tb Wipes or alcohol-based hand rub (ABHR). Allow to dry. 20. Incubate secondary plates in the designated area of the incubator in AFB room. 21. Replace the primary plates in biohazard bag and wipe bag with Oxivir Tb Wipe. 22. Label bag with pink Ebola stickers and keep in the incubator, in case needed for further work-up. H: AFB Room Clean Up: Note: Oxivir Tb Wipes require one minute contact time 1. Wearing full PPE, discard all disposable waste and any unused media into lined blue drum 2. Proceed with a primary clean: Wipe all surfaces inside the BSC and all surfaces used outside the BSC using Accel TB, including the surface of the disinfectant containers). Note the time and allow to remain wet 5 minutes, reapply if necessary 3. Perform a secondary clean by repeating the above process. 4. Wipe all the exterior surfaces of the blue drum with Oxivir Tb Wipes. 5. Place a blue doffing mat on the floor (white side up) and saturate with Accel TB. Place the blue drum on top of the mat. I: Waste removal from AFB room:
9 Page 9 of Remove PPE in the presence of an assistant, following doffing protocol and place all PPE into blue drum. Do not press down to compress. 2. Put on new gloves and a routine lab gown (gown is optional) 3. Tie off red bags, one inside the other, taking care to avoid contact with gown or self to the blue drum. 4. Clean gloves with Oxivir Tb Wipes or alcohol-based hand rub (ABHR). 5. Using the supplied silver marker, write the Quarantine Patient # (as issued by infection control) on the label provided. Place label on blue drum 6. Place two biohazard symbols on the blue drum so that they are visible from two sides. 7. Only if the patient s status is positive for Ebola, also affix the following provided labels so that they are visible from two sides of the drum: Two Infectious Substance Affecting Humans UN2814 Class 6.2 Two Incinerate Only 8. Place lid on drum. Aramark will clamp lid upon removal. 9. Remove routine gown if used and place in box provided for used AFB processing gowns. 10. Clean gloves with OxIvir Tb Wipe or Alcohol rub. Allow to dry. Using glove to glove technique remove gloves and place in routine biohazard waste. 11. Wipe surface of Oxivir Tb Wipes container and the ABHR container with an Oxivir Tb wipe 12. Carefully perform hand hygiene with ABHR or clean sink. 13. Leave AFB room, close door and secure room leaving signage in place and applying barrier (masking) tape over the door to prevent accidental entry. 14. Proceed according to the patient s current status: a. If the patient s Ebola status is still pending: - Call Aramark at and request Blue Drum removal for suspect Ebola Stat specialized terminal clean for Ebola
10 Page 10 of 13 b. If the patient s Ebola status is positive: - Call Aramark at Place request for: Blue Drum for positive Ebola Stat specialized terminal clean for Ebola 15. AFB room cannot be used until Aramark removes all waste and completes the final room cleaning. J: Day 2/ Secondary Inactivated Plates: If patient s status is pending, check with medical microbiologist for any updates before proceeding Ask the Medical Microbiologist to sign Waste Release Form 1. Wearing full PPE the AFB technologist will perform the Day 2 work up independently but will have an assistant for the donning and doffing following the lab protocol checklist. 2. Sign contact list for specimen processing. 3. Place absorbent material provided at the bottom of the blue bin then double the bin with red bags and place in AFB room. 4. Place sign on AFB room door Ebola containment / processing DO NOT ENTER. 5. Remove secondary plates from incubator and place into BSC. Confirm all plates are labelled with an inactivated sticker. 6. Wipe outside of plates with Oxivir Tb Wipe. 7. Check to confirm growth on secondary plates, repeat subculture from primary plates if necessary following the pm growth protocol. Remove inactivated plates from BSC. 8. Wipe all surfaces inside the BSC and all surfaces used outside the BSC using TB Accel, Allow to remain wet for 5 minutes 9. Wipe all the exterior surfaces of the blue drum with Oxivir Tb Wipes, then wipe the exterior surface of the Oxivir Tb Wipe containers 10. Place a blue doffing mat on the floor (white side up) and saturate with Accel TB. Place the blue drum on top of the mat. 11. Remove PPE in the presence of an assistant, following doffing protocol check list and place all PPE into blue drum.
11 Page 11 of Give secondary plates to the BC technologist. Secondary inactivated plates can be worked up on BC bench per usual procedures and using usual laboratory precautions. All inactivated labelled plates can be discarded per routine protocol. K: Waste removal from AFB room: Follow the waste removal protocol found in Section I, page 9, step 2 L: Day 3/ Examination of Anaerobic Plates If patient s status is pending, check with medical microbiologist for any updates before proceeding Ask the Medical Microbiologist to sign Waste Release Form Follow protocol as for G: Subculture of primary plates to secondary: Prepare supply bin with all required materials, including a new anaerobic jar and media plates for subculture of anaerobic organisms on primary plates to secondary plates. Include the protocols described in sections G and H and the doffing protocol (Appendix C), disposable loops, blue absorbent work surface mats, a double lined cardboard bucket for waste. Consider the gram stain results and the presence of any aerobic growth, allow for the possibility of more than one colony type being present. Include AFB (or designated) technologist will perform the work up independently but will have an assistant for the donning and doffing following the lab doffing protocol 1. Take all necessary supplies to the AFB Room 2. Place down blue absorbent mats and arrange all materials inside hood according to safe practice use of a BSC (instructions are posted near the BSC) 3. Sign the Infection Prevention sheet. 4. Don PPE with donning assistant 5. Place sign on door to AFB Room: Ebola Containment/Processing DO NOT ENTER
12 Page 12 of Place Open the anaerobic jar in the BSC and examine plates. 7. If anaerobic growth is suspected, follow the protocol in G for the subculture of primary plates to secondary plates. 8. Incubate secondary plates in a new anaerobic jar labelled with inactivated sticker. 9. Proceed with room cleanup as described in Section H (page 8) and the waste removal as in Section I (page 9) Subsequent work should follow the protocol listed in section J: Secondary plates, and the cleanup and waste removal as described in Sections H and I respectively.
13 Page 13 of 13 The regional medical discipline lead approves all new documents and any major changes. Draft versions of the microbiology documents are circulated regionally for input from other resources as required. Version Number Description of Change Date of Revision Reviewed By New Document (Prepared in collaboration with Infection Control, Medical Microbiologists and Laboratory leads) June Sidney Scharf Patricia Bleackley
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ]
![ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ] ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ]](/thumbs/78/77108755.jpg) Page 1 of 5 ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST Trained Observer: Unit: Date: Healthcare Worker: Assistant: Instructions: Check box after completion of each step. Donning of HEALTHCARE
Page 1 of 5 ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST Trained Observer: Unit: Date: Healthcare Worker: Assistant: Instructions: Check box after completion of each step. Donning of HEALTHCARE
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Competency. Method of Instruction Codes. Yes / No / RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN.
 JOHNS HOPKINS MEDICAL INSTITUTIONS Competency Validation Tool BIOLOGICAL PPE ENHANCED CONTACT AND DROPLET PRECAUTIONS (POWERED AIR PURIFYING RESPIRATOR (PAPR) OPTION) Employee Name: Role: Unit: Date: DESIRED
JOHNS HOPKINS MEDICAL INSTITUTIONS Competency Validation Tool BIOLOGICAL PPE ENHANCED CONTACT AND DROPLET PRECAUTIONS (POWERED AIR PURIFYING RESPIRATOR (PAPR) OPTION) Employee Name: Role: Unit: Date: DESIRED
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
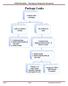 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015
 PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015 Personnel Maija Bluma (573-651-2069; mbluma@semo.edu) is the Microbiology Preparation
PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015 Personnel Maija Bluma (573-651-2069; mbluma@semo.edu) is the Microbiology Preparation
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
SPECIMEN COLLECTION. A Study Guide
 SPECIMEN COLLECTION A Study Guide Expectations At the UAP Marathon the UAP will be expected to verbalize the process of performing a peripheral blood draw and demonstrating the correct collection of a
SPECIMEN COLLECTION A Study Guide Expectations At the UAP Marathon the UAP will be expected to verbalize the process of performing a peripheral blood draw and demonstrating the correct collection of a
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Procedure 19 Changing A Clean Dressing. Procedure 20 Applying A Bandage. Procedure 21 Applying A Sterile Dressing
 Chapter 5 Wound Care Procedure 19 Changing A Clean Dressing Procedure 20 Applying A Bandage Procedure 21 Applying A Sterile Dressing Procedure 22 Applying A Dressing Around A Drain Procedure 23 Changing
Chapter 5 Wound Care Procedure 19 Changing A Clean Dressing Procedure 20 Applying A Bandage Procedure 21 Applying A Sterile Dressing Procedure 22 Applying A Dressing Around A Drain Procedure 23 Changing
Biomedical Laboratory Science
 Biomedical Laboratory Science!! " " New for 2015-2016 The 70% mastery for each skill has been added to the rating sheet so that competitors and advisors know what score is required for mastery. Rating
Biomedical Laboratory Science!! " " New for 2015-2016 The 70% mastery for each skill has been added to the rating sheet so that competitors and advisors know what score is required for mastery. Rating
Student Performance Guide. Student Performance Guide. Student Performance Guide
 LESSON 8-2 Collecting and Processing Specimens for Parasite Examination Student Performance Guide LESSON 8-3 Microscopic Methods for Student Performance Guide LESSON 8-4 Preparing and Staining Smears for
LESSON 8-2 Collecting and Processing Specimens for Parasite Examination Student Performance Guide LESSON 8-3 Microscopic Methods for Student Performance Guide LESSON 8-4 Preparing and Staining Smears for
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Scabies Identification, Treatment and Environmental Cleaning
 Scabies Identification, Treatment and Environmental Cleaning Level III Purpose The purpose of this procedure is to treat residents infected with and sensitized to Sarcoptes scabiei and to prevent the spread
Scabies Identification, Treatment and Environmental Cleaning Level III Purpose The purpose of this procedure is to treat residents infected with and sensitized to Sarcoptes scabiei and to prevent the spread
Original Date:
 Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System. Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe
 Chapter 6 Phlebotomy Procedure 29 Performing A Venipuncture Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe Procedure
Chapter 6 Phlebotomy Procedure 29 Performing A Venipuncture Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe Procedure
Standard Operating Procedure for disposal of biological waste
 Standard Operating Procedure for disposal of biological waste Effective date: 22 nd March 2018 Review due date: 19 th March 2018 Original Author Name: Rebecca Toone Position: Technician Date: 29.07.2013
Standard Operating Procedure for disposal of biological waste Effective date: 22 nd March 2018 Review due date: 19 th March 2018 Original Author Name: Rebecca Toone Position: Technician Date: 29.07.2013
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
This policy applies to all Pharmacy or non-pharmacy personnel accessing controlled work areas located within Lower Mainland Pharmacy Services.
 Page 1 of 13 BACKGROUND Proper hand hygiene and garbing are an essential part of the sterile compounding process to prevent patient harm from contaminated compounded sterile preparations and protect staff
Page 1 of 13 BACKGROUND Proper hand hygiene and garbing are an essential part of the sterile compounding process to prevent patient harm from contaminated compounded sterile preparations and protect staff
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
Introductory Chemistry
 Introductory Chemistry Lab 1: Introduction and Safety Objectives Learn how work to safely in the chemical laboratory Learn when and how to use the safety equipment in the chemical laboratory Learn the
Introductory Chemistry Lab 1: Introduction and Safety Objectives Learn how work to safely in the chemical laboratory Learn when and how to use the safety equipment in the chemical laboratory Learn the
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Policy: C-08-PRO Reviewed: 7/08
 Written: 1979 Policy: Reviewed: 7/08 Procedure Policy Manual Revised: 81, 84, 85, 86, 87, 88, 89, 90, 92, 93, 95 Ambulatory Care Division 2/97, 7/98, 7/00, 7/02, 7/04, 7/06, 2/09, 7/10 LSU Health Sciences
Written: 1979 Policy: Reviewed: 7/08 Procedure Policy Manual Revised: 81, 84, 85, 86, 87, 88, 89, 90, 92, 93, 95 Ambulatory Care Division 2/97, 7/98, 7/00, 7/02, 7/04, 7/06, 2/09, 7/10 LSU Health Sciences
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
PURPOSE: To define the responsibilities for obtaining nipple and breast milk cultures.
 1 PURPOSE: To define the responsibilities for obtaining nipple and breast milk cultures. RESPONSIBILITY STATEMENT: Staff may obtain nipple and breast milk culture with a physician order. SUPPORTIVE DATA:
1 PURPOSE: To define the responsibilities for obtaining nipple and breast milk cultures. RESPONSIBILITY STATEMENT: Staff may obtain nipple and breast milk culture with a physician order. SUPPORTIVE DATA:
Safe Handling and Disposal of Sharps. Reference Guide
 Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Hand Hygiene. Policy Title: Hand Hygiene Policy Number: 05. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Hazard Communication Program
 Purpose Hazard Communication The purpose of this Hazard Communication (Hazcom) is to provide a written procedure outlining Apache s system for handling hazardous chemicals/materials in the work place.
Purpose Hazard Communication The purpose of this Hazard Communication (Hazcom) is to provide a written procedure outlining Apache s system for handling hazardous chemicals/materials in the work place.
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Basic Microbiology and Immunology Practical Course
 Basic Microbiology and Immunology Practical Course 2 Lab # 2: Colouring the microorganisms Rules that must be followed to maintain an aseptic zone 3 For most bacterial cultures, you will use a sterile
Basic Microbiology and Immunology Practical Course 2 Lab # 2: Colouring the microorganisms Rules that must be followed to maintain an aseptic zone 3 For most bacterial cultures, you will use a sterile
Prepared by Laurel Arrigona, Matt Bavougian, Michael Crea, John Johnson, Steve Joyner, Sarah Robbin, and KC Stevenson
 122 nd AFDO Educational Conference Burlington, Vermont Body Art Committee June 10, 2018 Prepared by Laurel Arrigona, Matt Bavougian, Michael Crea, John Johnson, Steve Joyner, Sarah Robbin, and KC Stevenson
122 nd AFDO Educational Conference Burlington, Vermont Body Art Committee June 10, 2018 Prepared by Laurel Arrigona, Matt Bavougian, Michael Crea, John Johnson, Steve Joyner, Sarah Robbin, and KC Stevenson
CHAPTER 13 PROCEDURE checklists
 PROCEDURE checklists 179 CHAPTER 13 PROCEDURE checklists Procedure 13-1 Handwashing 1. Gather needed supplies if not present at the handwashing area: soap or the cleansing agent specified by your facility,
PROCEDURE checklists 179 CHAPTER 13 PROCEDURE checklists Procedure 13-1 Handwashing 1. Gather needed supplies if not present at the handwashing area: soap or the cleansing agent specified by your facility,
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
Table 6: Detailed Infection Prevention and Control Procedures for Tattooing and Micropigmentation. Use During Tattooing
 FACT SHEET Table 6: Detailed Infection Prevention and Control Procedures for and Micropigmentation 1. Skin Preparation Spray bottle with a solution of soap and water Single use disposable razor The skin
FACT SHEET Table 6: Detailed Infection Prevention and Control Procedures for and Micropigmentation 1. Skin Preparation Spray bottle with a solution of soap and water Single use disposable razor The skin
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
Ventricular Assist Device (VAD) Exit Site Care Guidelines
 Ventricular Assist Device (VAD) Exit Site Care Guidelines I. STATEMENT OF PURPOSE The Ventricular Assist Device (VAD) Exit Site Care Guidelines are intended to provide standardization and continuity of
Ventricular Assist Device (VAD) Exit Site Care Guidelines I. STATEMENT OF PURPOSE The Ventricular Assist Device (VAD) Exit Site Care Guidelines are intended to provide standardization and continuity of
Infection Prevention Guidelines. Safe Use, Handling & Disposal of Sharps
 Infection Prevention Guidelines Safe Use, Handling & Disposal of Sharps Author: Anne Tateson Owner: Infection Prevention Team Publisher: Infection Prevention Date of Version Issue: February 2015 Version:
Infection Prevention Guidelines Safe Use, Handling & Disposal of Sharps Author: Anne Tateson Owner: Infection Prevention Team Publisher: Infection Prevention Date of Version Issue: February 2015 Version:
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Dixie State University
 CDCA Host Site Guide March 31, 2018 Dixie State University Exam Site Information for Candidates CDCA Examination March 31, 2018 Dixie State University TAYLOR HEALTH SCIENCES BUILDING 1526 MEDICAL CENTER
CDCA Host Site Guide March 31, 2018 Dixie State University Exam Site Information for Candidates CDCA Examination March 31, 2018 Dixie State University TAYLOR HEALTH SCIENCES BUILDING 1526 MEDICAL CENTER
Student Performance Guide. Student Performance Guide. Student Performance Guide. Student Performance Guide. LESSON 3-3 Bleeding Time
 LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
Environmental Health Department 58 St Johns Road, Newport, Isle of Wight PO30 1LT
 Environmental Health Department 58 St Johns Road, Newport, Isle of Wight PO30 1LT 2 The Local Government (Miscellaneous Provisions) Act 1982 requires persons who practice acupuncture, tattooing, ear piercing
Environmental Health Department 58 St Johns Road, Newport, Isle of Wight PO30 1LT 2 The Local Government (Miscellaneous Provisions) Act 1982 requires persons who practice acupuncture, tattooing, ear piercing
PharmChek Drugs of Abuse Sweat Patch Training Manual
 PharmChek Drugs of Abuse Sweat Patch Training Manual For the Application, Removal, Specimen collection, and Transport of the PharmChek Drugs of Abuse Patch For Professional Use Only Revised November, 2016
PharmChek Drugs of Abuse Sweat Patch Training Manual For the Application, Removal, Specimen collection, and Transport of the PharmChek Drugs of Abuse Patch For Professional Use Only Revised November, 2016
PREPARATION OF BLOOD FILMS FOR MALARIA DETECTION
 PREPARATION OF BLOOD FILMS FOR MALARIA DETECTION Materials for Preparation of Malaria Smears: Clean and wrapped slides Sterile lancets 70% ethanol and water Absorbent cotton wool Surgical gloves Lint-free
PREPARATION OF BLOOD FILMS FOR MALARIA DETECTION Materials for Preparation of Malaria Smears: Clean and wrapped slides Sterile lancets 70% ethanol and water Absorbent cotton wool Surgical gloves Lint-free
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control
 The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control Author University Health Service, The University of Hong Kong Approved By Task Force on Infectious Diseases,
The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control Author University Health Service, The University of Hong Kong Approved By Task Force on Infectious Diseases,
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
LYMPHOCYTE ISOLATION
 Blood tubes to be processed for Lymphocyte Isolation and/or Lymphocyte Transformation should be placed in the rack located on top of the black cabinet and stored at room temperature. A. WARM WASH MEDIUM
Blood tubes to be processed for Lymphocyte Isolation and/or Lymphocyte Transformation should be placed in the rack located on top of the black cabinet and stored at room temperature. A. WARM WASH MEDIUM
LAB 5 Blood Collection
 LAB 5 Blood Collection Purpose The Purpose of this lab is to learn how to collect a sample of blood properly. From this blood sample disease such as anemia, viral infections, iron deficiency, spherocytosis,
LAB 5 Blood Collection Purpose The Purpose of this lab is to learn how to collect a sample of blood properly. From this blood sample disease such as anemia, viral infections, iron deficiency, spherocytosis,
Procedure/ Care Plan for Domiciliary Care Workers/ Support Workers - Application of Prescribed Creams/ Ointments/ Lotions (Adult)
 Application of Prescribed Creams/ Ointments/ Lotions (Adult) CLINICAL GUIDELINES ID TAG Medicines Management Specific Title: Procedure: Application of prescribed Creams/ Ointments/ Lotions (Adult) Author:
Application of Prescribed Creams/ Ointments/ Lotions (Adult) CLINICAL GUIDELINES ID TAG Medicines Management Specific Title: Procedure: Application of prescribed Creams/ Ointments/ Lotions (Adult) Author:
BTB000 Manufacturers Product Code: TAB/R/DR
 BTB000 Manufacturers Product Code: TAB/R/DR Red Polythene Drug Round Tabard (21 x 24 / 30mu) black print Drug Round in Progress. One size fits all. Drug round tabards improve the efficiency and accuracy
BTB000 Manufacturers Product Code: TAB/R/DR Red Polythene Drug Round Tabard (21 x 24 / 30mu) black print Drug Round in Progress. One size fits all. Drug round tabards improve the efficiency and accuracy
Bacterial smear and Staining
 Practical Microbiology 18-22/11/2018 University of Sulaimani college of Pharmacy Year2 Lab. 4: Bacterial smear and Staining Before staining and observing a microbe under a microscope, a smear must be prepared.
Practical Microbiology 18-22/11/2018 University of Sulaimani college of Pharmacy Year2 Lab. 4: Bacterial smear and Staining Before staining and observing a microbe under a microscope, a smear must be prepared.
Medical Waste Management Plan
 Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
