x. ANNUAL REVIEW SIGNATURE SHEET
|
|
|
- Rhoda Little
- 5 years ago
- Views:
Transcription
1 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by the revised date. Medical Director Signature/Date:
2 L,; :"w"r/' <;~~~ _~,,*);,~:J:\:'i~}"c~.if:,2';~~~:;'~'?j,f''':,\~ :,: S'"~~:-J::;3; ::. ;~_~~, ~j'<t{; :"2r""..;, -;_~~~":; : - 1\~ 1" tas~~s ;, whi,c ti:, il~tpl:iide ' -rem.6ya~1. -or~, recapp:i ng ~i~':~} ' ~~~ :,: of ~ a -' needl'e.. from a '_ syringe', ejt adapto'r ar_e <:,}hi,!:,,;';/l~? ~fl~f~jii~iflf:~ ~tii~~i~~~i~~i~!~1t~,~tt~~&fr< UNIVERSAL PRECAUTIONS It is the policy of University Hospital that all employees will utilize Universal Precautions U.P.) principles of infection control in their periormance of direct and indirect patient care. 1 All laboratory workers will follow the hospital policy PI-12 on universal precautions see attached to this policy) and, in addition, will follow the specific recommendations outlined below. It is the responsibility of each supervisor to provide training for employees on U.P. procedures. It is the responsibility of each employee to practice U.P. at all times. See also lab safety policy P-1 0, Personal Protective Equipment. A. Agenda to Hospital Policy PI Hand Washing 2 Hands should be washed whenever there is visible contamination with blood or body fluids, after completion of work, and before leaving the laboratory, after removing gloves, before eating, drinking, smoking, applying makeup, changing contact lenses, and using lavatory facilities, and before any and all other activities which involve contact touching) of the hand with mucous membranes, eyes, or breaks in the skin. If contact of blood, body fluids, or tissue occurs because of a break hole or tear) in the glove, the gloves should be immediately removed and the hands thoroughly washed. Washing with soap and water is recommended. No additional benefit has been established for routine washing with antiseptic soaps or antiseptics 2 Use of a moisturizing hand cream after washing may reduce skin irritation caused by frequent hand washing. A hand washing sink should be designated in each laboratory work area. It should be separate from sinks used for washing equipment or for waste disposal Needles and Sharps2 a. All tasks in which needles or sharps are used pose a risk to the health care worker. Specific operating procedures for their use are outlined in the department procedure manuals. b. The use of needles in the laboratory is discouraged except for phlebotomy or when no other alternative is feasible. Although the recapping and removal of needles is also strongly discouraged, there are certain procedures periormed in the laboratory for which recapping of the needle or removal of the needle is unavoidable. Any task in which a needle must be removed or recapped is considered a high-risk task, and special precautions must be taken. Other tasks in which there is significant manipulation of needles are also of particular risk.
3 r, c. High risk tasks include Handling Procedure Removal of vacutainer needles from adaptor Removal of uncapped needle from syringe Use one-handed technique to place needle into the needle jig on the sharps container. Unscrew the adaptor and let needle drop into sharps container. DO NOT pick up sharps container in other hand to perform this task. Use one-handed technique to place needle into the needle jig on the sharps container. Unscrew the syringe and let needle drop into sharps container. Alternatively, use one-handed technique to re-cap needle. Use pliers to brace the capped needle and slowly twist off the syringe with the other hand. Capping a syringe needle Removal of capped needle from syringe Use one-handed technique to slide needle into cap. Then use second hand to tighten cap. Whenever possible, use pliers to brace the capped needle and twist off the syringe with the other hand. If situation is such that pliers cannot be used, hold capped needle in one hand and slowly twist the syringe away from the needle with the other hand. DO NOT let the needle slide out of the cap. Transfer of blood specimen from a syringe to an evacuated tube Puncture the diaphragm of the rubber stopper and allow the correct amount of fluid. The vial should not
4 be hand held when puncturing the top. Handling butterfly adapters Handle by wings only and dispose of directly into a sharps container. Do not attempt to remove a vacutainer adaptor from a butterfly. 3. Protective Barriers A. Gloves Latex or vinyl gloves must be worn for all procedures involving capillary puncture, phlebotomy, and arterial and bone marrow specimen collection. Latex or vinyl gloves must be worn 2 by all personnel who handle or process tissues, blood, serum, plasma, cerebrospinal fluid, vaginal secretions, semen, bronchopulmonary washings, synovial fluid, peritoneal fluid, pleural fluid, amniotic fluid, pericardial fluid, or any other bodily material visibly contaminated with blood. Since saliva and breast mild contain low concentrations of HBV and HIV in the absence of visible blood contamination, gloves are recommended when handling specimens of saliva, sputum, and breast milk. 2 'I ) Latex or vinyl gloves should be worn when handling items that are likely to be contaminated,2 including biological safety cabinets, laboratory instruments, specimen containers, counter tops, computer keyboards, and telephones. These items should all be considered contaminated if in the laboratory setting, so hand washing is recommended before leaving the area. Latex or vinyl gloves must be worn when handling biohazard bagged material and visibly contaminated items or linen 2 and also for biohazard spill cleanup. Latex or vinyl gloves are available to all employees for use. If any employee experiences allergies to a particular glove product, a reasonable effort will be made to find glove liners or a non-allergenic product for the employee to wear. Gloves should be changed if they accidently become visibly contaminated with blood or body fluids, if physical damage tears or holes) occurs, or if chemical damage e.g., from organic solvents) occurs. b. Gowns Long laboratory coats must be worn when handling specimens. Lab coats must be buttoned. If the laboratory coats cannot be buttoned, an apron or gown must be worn. Laboratory coats used during open specimen handling or testing cannot be worn outside the laboratory area or for phlebotomy. Unsoiled laboratory coats used during phlebotomy may be used while working in the laboratory. If laboratory coats are worn in non-clinical areas of the institution, coats must not have been in use for specimen handling or testing or for phlebotomy. Laboratory coats are
5 r not required in non-clinical areas. If infectious material is spilled on a laboratory coat, remove promptly, place in a red bag, and place bag in the lab laundry hamper. Lab coats may not be taken home and laundered by the lab employee. If infectious material is spilled on personal clothing, remove clothing as soon as possible. The personal clothing must be decontaminated before the employee can take it home. The use of an anti-viral agent such as Virex is recommended in a warm water soak. Once decontaminated, personal clothing can be taken home and cleaned with household laundry detergent. Gowns or aprons made of impervious materials, e.g., plastic-paper alloys, should be worn during procedures known or suspected to result in splashing of body fluids. c. Masks, Eyewear Masks and protective eyewear or face shields must be worn for blending, sonicating, vigorous mixing, and when removing tightly impacted stoppers from tubes - or in any other situation where the health care worker anticipates mucous membrane contamination. d. Occlusive Dressings All skin defects e.g., exudative lesions, dermatitis, cuts, or abrasions) located on parts of the body exposed to blood or body fluid should be covered with a waterimpermeable occlusive bandage or otherwise shielded. This includes defects on the arms, face, and neck Mouth to Mouth Resuscitation - no additions to hospital policy. 5. Employee Specifics a. Smoking, eating, and drinking are not allowed in the laboratory area. Contact lenses should not be inserted or removed in the laboratory area. Application of cosmetics, lip balm, etc., is not allowed in the laboratory area. b. Fingers, pencils, and other objects used in the laboratory must be kept away from the mouth and/or mucous membranes. c. Employees failing to comply with safety practices or who endanger the safety of other employees, patients, or visitors will receive a corrective action. Employees who do not immediately correct their actions will receive a disciplinary action, which may result in loss of payor dismissal. 6. Environmental b. Spills continued)
6 Wear gloves and a gown to clean all spills. If the spill contains broken glass or other objects, these should be removed without contact with the hands. Rigid sheets of cardboard used as a 'pusher' and "receiver" may be used to handle such objects and then discarded into the biohazard waste container. In a centrifuge, a forceps with gauze can be used to remove bits of glass. If the spill is large and/or there is potential of contaminating the workers' shoes, waterimpermeable shoe covers should be worn. r Absorb the spill with disposable absorbent material e.g., paper towel, gauze pads, or tissue wipes). Note - the spill cannot be adequately disinfected unless the bulk of it is absorbed and picked up first. Clean the spill site of all visible spilled material using an aqueous detergent solution. Absorb the liquid. Disinfect the spill site using an appropriate intermediate to high-level hospital disinfectant, e.g., 1:10 bleach. Flood the spill site or wipe down the spill site until it is "glistening wet.' Let bleach site on the site for a minimum of 2 minutes. For other disinfectants, follow manufacturer's directions.) Absorb the disinfectant solution with disposable material. Rinse with water to remove any noxious chemicals or odors. Dry the site. Dispose of solid materials in a biohazard waste container. Use an intermediate rigid container if the spill contained sharps. Large quantities of liquid can be disposed of in a san itary sewer. f. Pipetting g. Mouth pipetting is not allowed. All pipetting must be performed using a rubber bulb or mechanical device. Centrifugation All specimens must be capped during centrifugation and contained within aerosol
7 r protection/containment cups or a closed rotor. The centrifuge lid or rotor should not be opened wh ile the centrifuge is running. h. Biohazard Containers Sharps contains, small waste containers, and biohazard waste boxes must be provide in all work areas. i. Specimen Analysis All testing procedures in which U.P. fluids are assayed must include the use of protective barriers appropriate to the tasks. j. Disinfecting Work Areas/Equipment Work surfaces should be cleaned and disinfected at the end of each work shift. Equipment should be cleaned and decontaminated prior to being repaired or transported to a contracted repair service. k. Food Storage r Food may be stored in a designated refrigerator or in a designated office area. A designated refrigerator must contain a prominent sign on the outside stating "Store only food in this refrigerator." Refrigerators not designated for food must contain a prominent sign on the outside stating "Do not store food in this refrigerator." A designated office area is one which has been identified as a clean area and in which no gloves or contaminated lab coats are worn. 7. Specimen Handling a. Requisitions and Specimen Containers Requisitions or specimen containers contaminated with blood or body fluids should be placed in a bag labeled biohazardous or red and the originating nursing unit called to clean the container with approved disinfectant and to replace the lab requisition form. If a representative from the originating unit does not clean the requisition and container within 15 minutes, the plastic bag should be returned to the unit The lab will not accept or process specimens with exposed needles. The lab will not accept urine or fluids in paper containers. The primary specimen container for all specimens must be leakproof. For U.P. fluids, each specimen must be placed inside a secondary container for transport. The secondary container can be a plastic bag, a secondary test tube, or a test tube rack in a plastic tub or phlebotomy tray.
8 b. Retumed units of blood must be in a secondary container, e.g., a plastic bag. Specimen Handling All specimens should be covered, capped, corked, or plugged except while being separated, poured, or during analysis. To remove a rubber stopper from a tube, use plastic coated gauze to cover the stopper or work behind an acrylic splash shield. All microbiology specimens should be opened in a biosafety hood. All containers of bronchopulmonary washings or sputum should be handled as if they are contaminated on the outside. 2 B. Hepatitis Immunization Hepatitis B vaccine is available to all employees of the clinical laboratory through University Hospital Health Services. The vaccine will be offered to all employees on employment see UH policy HH-7) but may be received at any point in employment. C. Accident Reporting References See lab policy on accident reporting and hospital policy on blood exposure UH Policy HB-1). 1. Infection Control/Universal Precautions, UH Policy PI-12, Oct. 30, Protection of laboratory Workers from Infectious Disease Transmitted by Blood, Body Fluids, and Tissue, 2nd Ed., NCClS Document M29-T2, Vol. 11, No. 14, 1991.
9 ,. \ 1 2 Attachments Infection Control/Universal Precautions, UH Policy PI-12, Oct. 30, This policy must be followed in addition to the laboratory policy above. NCClS Document M29-T2: Protection of laboratory Workers, p This information on the epidemiology, transmission of human hepatitis viruses and retroviruses, and survival of HIV, is required reading for all laboratory workers who fall under universal precautions.
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
What is infection control?
 Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
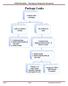 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Original Date:
 Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
SKIDMORE COLLEGE. Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS. Introduction -- Pg. 2
 SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Bloodborne Pathogens Safety in Your Workplace
 Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Safe Handling and Disposal of Sharps. Reference Guide
 Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
LAB 5 Blood Collection
 LAB 5 Blood Collection Purpose The Purpose of this lab is to learn how to collect a sample of blood properly. From this blood sample disease such as anemia, viral infections, iron deficiency, spherocytosis,
LAB 5 Blood Collection Purpose The Purpose of this lab is to learn how to collect a sample of blood properly. From this blood sample disease such as anemia, viral infections, iron deficiency, spherocytosis,
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS
 SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS Purpose To ensure the safe disposal of potentially contaminated sharps, syringes, clothing and any other waste products. Scope
SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS Purpose To ensure the safe disposal of potentially contaminated sharps, syringes, clothing and any other waste products. Scope
BLOODBORNE PATHOGENS
 MARICOPA COUNTY SHERIFF S OFFICE POLICY AND PROCEDURES Subject Related Information CRITICAL POLICY PURPOSE BLOODBORNE PATHOGENS Supersedes CP-6 (08-14-15) Policy Number CP-6 Effective Date 11-22-16 The
MARICOPA COUNTY SHERIFF S OFFICE POLICY AND PROCEDURES Subject Related Information CRITICAL POLICY PURPOSE BLOODBORNE PATHOGENS Supersedes CP-6 (08-14-15) Policy Number CP-6 Effective Date 11-22-16 The
Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System. Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe
 Chapter 6 Phlebotomy Procedure 29 Performing A Venipuncture Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe Procedure
Chapter 6 Phlebotomy Procedure 29 Performing A Venipuncture Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe Procedure
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN
 ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
Environmental Health Department 58 St Johns Road, Newport, Isle of Wight PO30 1LT
 Environmental Health Department 58 St Johns Road, Newport, Isle of Wight PO30 1LT 2 The Local Government (Miscellaneous Provisions) Act 1982 requires persons who practice acupuncture, tattooing, ear piercing
Environmental Health Department 58 St Johns Road, Newport, Isle of Wight PO30 1LT 2 The Local Government (Miscellaneous Provisions) Act 1982 requires persons who practice acupuncture, tattooing, ear piercing
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Table 5: Detailed Infection Prevention and Control Procedures for Body Piercing. drape the piercing site.
 FACT SHEET Table 5: Detailed Infection Prevention and Control Procedures for Body Piercing Equipment / Single use towel 1. Client preparation A towel may be used to drape the piercing site. The towel should
FACT SHEET Table 5: Detailed Infection Prevention and Control Procedures for Body Piercing Equipment / Single use towel 1. Client preparation A towel may be used to drape the piercing site. The towel should
Student Performance Guide. Student Performance Guide. Student Performance Guide. Student Performance Guide. LESSON 3-3 Bleeding Time
 LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
Table 6: Detailed Infection Prevention and Control Procedures for Tattooing and Micropigmentation. Use During Tattooing
 FACT SHEET Table 6: Detailed Infection Prevention and Control Procedures for and Micropigmentation 1. Skin Preparation Spray bottle with a solution of soap and water Single use disposable razor The skin
FACT SHEET Table 6: Detailed Infection Prevention and Control Procedures for and Micropigmentation 1. Skin Preparation Spray bottle with a solution of soap and water Single use disposable razor The skin
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
rooo.lb IOWA COUNTY ORDINANCE NO TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS:
 .. rooo.lb IOWA COUNTY ORDINANCE NO. 4-196 TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS: SECTION I: The following ordinance of Iowa County, Wisconsin is hereby created
.. rooo.lb IOWA COUNTY ORDINANCE NO. 4-196 TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS: SECTION I: The following ordinance of Iowa County, Wisconsin is hereby created
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
LYMPHOCYTE ISOLATION
 Blood tubes to be processed for Lymphocyte Isolation and/or Lymphocyte Transformation should be placed in the rack located on top of the black cabinet and stored at room temperature. A. WARM WASH MEDIUM
Blood tubes to be processed for Lymphocyte Isolation and/or Lymphocyte Transformation should be placed in the rack located on top of the black cabinet and stored at room temperature. A. WARM WASH MEDIUM
Cherie Maguire, MS Research Assistant II Channing Division of Network Medicine. The Center for Clinical Investigation
 Cherie Maguire, MS Research Assistant II Channing Division of Network Medicine The Center for Clinical Investigation What is Phlebotomy Safety Supplies and Equipment Patient Identification/Patient Preparation
Cherie Maguire, MS Research Assistant II Channing Division of Network Medicine The Center for Clinical Investigation What is Phlebotomy Safety Supplies and Equipment Patient Identification/Patient Preparation
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
Surgical Gown. Tongue Depressor. A disposable gown worn by medical staff during surgery. A thin, flat, wooden stick rounded at both ends
 Tongue Depressor A thin, flat, wooden stick rounded at both ends Accidentally dropped on the floor by the doctor 16 Surgical Gown A disposable gown worn by medical staff during surgery Used by the surgeon
Tongue Depressor A thin, flat, wooden stick rounded at both ends Accidentally dropped on the floor by the doctor 16 Surgical Gown A disposable gown worn by medical staff during surgery Used by the surgeon
TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION
 TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION 1. GENERAL PRACTITIONER INFORMATION New Registration Annual Registration Updated Registration FULL LEGAL NAME (Give
TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION 1. GENERAL PRACTITIONER INFORMATION New Registration Annual Registration Updated Registration FULL LEGAL NAME (Give
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Safe Sharps Disposal. Learn how to safely dispose of used sharps including needles, lancets and syringes. Expanded Syringe Access Program
 Safe Sharps Disposal Learn how to safely dispose of used sharps including needles, lancets and syringes. Expanded Syringe Access Program How to Safely Dispose of Household Sharps Millions of people use
Safe Sharps Disposal Learn how to safely dispose of used sharps including needles, lancets and syringes. Expanded Syringe Access Program How to Safely Dispose of Household Sharps Millions of people use
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
Hand Hygiene. Policy Title: Hand Hygiene Policy Number: 05. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
Hygienic requirements for tattoo and piercing studios
 Hygienic requirements for tattoo and piercing studios Activities injuring the skin or mucus membrane are linked to an increased infection risk for diseases transferred by blood and serum. To avoid transferable
Hygienic requirements for tattoo and piercing studios Activities injuring the skin or mucus membrane are linked to an increased infection risk for diseases transferred by blood and serum. To avoid transferable
