(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
|
|
|
- Abigail McDonald
- 5 years ago
- Views:
Transcription
1 (19) United States US 2012O095206A1 (12) Patent Application Publication (10) Pub. No.: US 2012/ A1 CHEN et al. (43) Pub. Date: (54) METHOD FOR PRODUCING CROSS-LINKED Publication Classification HYALURONCACD (51) Int. Cl. (75) Inventors: TOR-CHERN CHEN, Kaohsiung e Zee 30.8 SEW LI-SUCHEN, Kaohsiung (52) U.S. Cl /56; 536/123.1 (57) ABSTRACT (73) Assignee: SIYSION BIOTECH INC., A method for producing a cross-linked hyaluronic acid in.e.p.z. (TW) accordance with the present invention comprises: (a) cross linking at least one polymer at a temperature, from about 35 (21) Appl. No.: 13/316,840 C. to about 60 C., for a reaction time of from about 0.1 hour 1-1. to about 72 hours with a cross-linking agent, and (b) lowering (22) Filed: Dec. 12, 2011 the temperature in step (a) to form about 10 C. to about 30 O O C. for a reaction time of from about 48 hours to about 28 days Related U.S. Application Data to obtain the cross-linked hyaluronic acid, whereby, a cross (63) Continuation-in-part of application No. 12/385,502, linking agent content in a product of the method can be filed on Apr. 9, decreased so the product does not require purification.
2 US 2012/ A1 METHOD FOR PRODUCING CROSS-LINKED HYALURONIC ACID CROSS REFERENCE TO RELATED APPLICATIONS This nonprovisional utility application is a continu ation-in-part of and claims the benefit under 35 U.S.C. S 120 to co-pending U.S. application Ser. No. 12/385,502 filed Apr. 9, 2009, all of which are incorporated, in their entirety, by this reference. BACKGROUND OF THE INVENTION Field of the Invention 0003) The present invention relates to a method for pro ducing a cross-linked hyaluronic acid, especially producing cross-linking hyaluronic acid with decreased cross-linking agent content Description of the Prior Arts 0005) Hyaluronic acid is a kind of polysaccharides, which is composed of disaccharides and 400D of molecular weight. The disaccharide is composed of B-1,4-glucuronic acid and B-1,3-N-acetylglucosamine linked together by B-1.4 glyco sidic bond. Moreover, the disaccharide links other disaccha rides by B-1.3 glycosidic bonds to form linear polysaccha ride. Currently, hyaluronic acid is synthesized by bacteria Such as Streptococcus and is obtained by extracted from ani mal tissues such as from cockscomb Since hyaluronic acid, hyaluronate and derivatives thereof have good biocompatibility, bio-degradability and Viscoelasticity, they can be used in cosmetics, biomedicine, medical products and pharmaceutical industry Since the organism of linear hyaluronic acid can be easily degraded by enzymes, such as hyaluronidase and free radicals, residence time of the organism of linear hyaluronic acids is relevantly shorter. Furthermore, linear hyaluronic acid has low mechanical strength, so the linear hyaluronic acid has limited applications. Therefore, the cross-linked hyaluronic acid is most preferably used When practicing the operating method, the cross linked hyaluronic acid have different type to the different goal. Such as a solution type, a hydrogel type, a matter between a solution type and hydrogel type, and a mixture consisting of a solution type and a hydrogel type. A manu facture method for producing the cross-linked hyaluronic acid comprises: mixing a cross-linking agent and hyaluronic acid to proceed a cross-linking reaction and to obtain the cross-linked hyaluronic acid; and purifying the cross-linked hyaluronic acid to remove the excess cross-linking agent. The Step of purifying the cross-linked hyaluronic acid may be by dialysis or washing with water or a buffer solution. However, the above-mentioned dialysis or washing cannot remove all of the residual cross-linking agent, especially because it has a bonding-state end and a free-state end. Furthermore, the free State end of the cross-linking agent is still active and can create undesirable side effects when applied in animals, as shown by U.S. Pat. No. 5,808, Also, the manufacture method for purifying the cross-linked hyaluronic acid by dialysis or washing also faces the following difficulties: 0010) 1. Refining cannot be easily scaled up at an industry level. 0011) 2. The cross-linked hyaluronic acid requires sterile conditions; otherwise, contaminants can easily be integrated into a final product. However, the manufacture method for the cross-linked hyaluronic acid by dialysis or washing is under neutral or almost neutral condition, so sterile conditions are almost always difficult to be controlled. I When the cross-linked hyaluronic acid is hydro gel and when the cross-linked hyaluronic acid with low degree of cross-linking swells significantly, the cross-linked hyaluronic acid is difficult to be washed and the cross-linked hyaluronic acid may be easily lost during the washing pro cess. Similarly, when the cross-linked hyaluronic acid with high degree of cross-linking swells insignificantly, the cross linking agent may not be capable of being removed with ease. I Moreover, the cross-linked hyaluronic acid with low degree of cross-linking can be easily washed away when washing the cross-linked hyaluronic acid, so that the residual cross-linked hyaluronic acid has decreased its lubrication. Therefore, when the cross-linked hyaluronic acid is used for injection, straight-chain or cross-linked hyaluronic acid solu tion has to be added in the residual cross-linked hyaluronic acid to increase its lubrication U.S. Pat. No. 4,716,154 discloses a manufacture method for a gel of cross-linked hyaluronic acid for use as a Vitreous humor substitute, comprising: cross-inking a hyalu ronate with multifunctional cross-linking agent at 50 C. for two hours under alkaline condition and placing overnight at room temperature to obtain a gel; for removing un-reacted cross-linking agent, cutting the gel into small pieces and Washing thoroughly for 24-hours using distilled water and further washing for 8 hours by boiled saline water to obtain gel with 0.23% to 1.2% of solid content. However, the fore going method has the following shortcomings: I0015 (1) a complex purifying step is required; 0016 (2) the gel can swell and the gel content is low when removing cross-linking agent with boiled saline water and attaining a gel form. Therefore, the process needs extra step to improve the gel content; and (3) the cross-linked hyaluronic acid has to swell again in the buffer solution to adjust the osmotic pressure and the ph value so that the method is unsuitable to be conducted at an industry level. I0018 US 2006/ A1 discloses cross-linking of low and high molecular weight hyaluronic acids to obtain monophase hydrogels. The monophase hydrogels have good mechanical strength with improved properties for injection. However, the monophase hydrogels have residual cross-link ing agent over 300 ppm after cross linking reaction at 50 C. Then, the residual cross-linking agent is intended to be removed by dialysis but the cross-linking agent cannot be removed thoroughly. Therefore, neither un-reacted cross linking agent with two free-state ends nor reacted cross linking agent with one free-state end and one bonding-state end in the hydrogels can be removed by dialysis or washing. I0019 US 2005/ A1 discloses methods for making injectable polymer hydrogels, comprising: (a) cross-linking one or more polymers to form a gel, (b) washing the gel, (c) purifying the gel, and (d) homogenizing the gel to produce the hydrogel. The method uses a di-functional or multifunctional cross-linking agent with high concentration to manufacture hydrogel. Therefore, the above-mentioned hydrogel contains residual cross-linking agents. Moreover, the hydrogel needs 2 to 3 days to wash and to purify the gel. However, the hydrogel is under almost neutral condition and may be polluted by microorganism.
3 0020 US 2007/ A1 discloses a method for manu facturing a cross-linked polysaccharide composition com prising: (a) contacting a polysaccharide mixed in an alkaline medium with a bifunctional or polyfunctional epoxide to provide an essentially epoxy cross-linked polysaccharide, wherein the epoxide is substantially linked to the polysaccha ride by ether bonds; (b) drying the epoxy cross-linked polysaccharide without Substantially removing epoxide from the alkaline medium to form a cross-linked polysaccharide matrix; (c) optionally washing the cross-linked polysaccha ride matrix with a water miscible solvent; and (d) neutralising the cross-linked polysaccharide matrix with an acidic medium to form a cross-linked polysaccharide gel. There fore, the method delivers a product having residual cross linking agents U.S. Pat. No. 4, discloses a method of manufacturing cross-linked hyaluronic acid using precipita tion to remove the cross-linking agent. However, the method produces a product that still contains residual cross-linking agents When cross-linking polymers to form a gel in an alkaline medium, the cross-linking reaction competes with a hydrolysis reaction as shown by Y. Tokita and A Okamoto, Hydrolytic Degration of Hyaluronic Acid', Polymer Degra tion and Stability, vol. 48, pp (1995). In the initial reaction period, the concentration of the cross-linking agent is high, so the cross-linking reaction is the principal reaction. But, after a specific amount of the cross-linking agent is consumed, the hydrolysis reaction becomes the principal reaction and destroys the gel causing gel degradation. There fore, the cross-linking reaction in an alkaline medium has to be terminated before Such secondary reaction occurs, despite the fact that the gel still contains much residual cross-linking agent. The manufacturing method comprises: cross-linking a hyaluronate and di-functional or multi-functional cross-link ing agent in 25 to 60 C. for a period of between 10 minutes and 24 hours under basic condition to obtain a gel; and puri fying the gel to remove cross-linking agents. However, the cross-linking agent cannot be removed thoroughly. An actual reaction time depends on the temperature and the basic con dition. No matter how the gel obtained in foregoing methods intend to achieve purification, a certain/significant amount of cross-linking agents still remains in the cross-linked hyalu ronic acid WO-00/46253 A1 discloses a process for the pro duction of multiple cross-linked hyaluronic acid derives. The process comprises two cross-linking reactions at an uniform temperature, and the cross-linking agents, feeding ratios, and/ or ph values of the two cross-linking reactions are different. However, the cross-linking agents with free-state functional group in the cross-linked hyaluronic acid obtained thereby is quite high and the cross-linked hyaluronic acid cannot be applied to a human or an animal directly. A washing step and/or a purifying step is necessary for obtaining a physi ologically acceptable cross-linked hyaluronic acid where the residual cross-linking agent in the product is over 300 ppm A preparation of cross-linked hyaluronic acid is also disclosed by Tomihata and Ikada (Biomaterials, 1997, 18, ). A strategy to remove a cross-linking agent with free-state functional group provided is elongation of the reac tion time. However, a washing step and/or a purifying step is necessary for obtaining a physiologically acceptable cross linked hyaluronic acid where the residual cross-linking agent in the product is over 300 ppm. Furthermore, a deterioration in the cross-linked hyaluronic acid occurs that causes the cross-linked hyaluronic acid to turn deep yellow or deep brown or cause a final product to degrade obviously To overcome the shortcomings, the present inven tion provides a method for producing a cross-linked hyalu ronic acid to mitigate or to obviate the aforementioned prob lems. SUMMARY OF THE INVENTION The present invention provides a method for pro ducing a cross-linked hyaluronic acid with a lower level of cross-linking agent A method for producing a cross-linked hyaluronic acid in accordance with the present invention comprises: 0028 (a) cross-linking at least one polymer at a tempera ture, from about 35 C. to about 60 C., for a reaction time of from about 0.1 hour to about 72 hours with a cross linking agent; and 0029 (b) lowering the temperature in step (a) to form about 10 C. to about 30 C. for a reaction time of from about 48 hours to about 28 days to obtain the cross-linked hyaluronic acid The steps (a) and (b) are conducted under a basic condition The polymer is selected from the group consisting of hyaluronic acid, hyaluronate, carboxymethylcellulous (CMC), alginate, chondroitin-4-sulfate, chondroitin-6-sul fate, Xanthane gum, chitosan, pectin, agar, carrageenan and guar gum thereof and a mixture thereof The method is absent a step of purifying the cross linked hyaluronic acid and the content of the cross-linking agent with free-state functional group is below about 300 ppm in the cross-linked hyaluronic acid Other objectives, advantages and novel features of the invention will become more apparent from the following detailed description. DETAILED DESCRIPTION OF THE INVENTION A method for producing a cross-linked hyaluronic acid in accordance with the present invention comprises: 0035 (a) cross-linking at least one polymer at a tempera ture, from about 35 C. to about 60 C., for a reaction time of from about 0.1 hour to about 72 hours with a cross linking agent; and 0036 (b) lowering the temperature in step (a) to from about 10 C. to about 30 C. for a reaction time of from about 48 hours to about 28 days to obtain the cross-linked hyaluronic acid The steps (a) and (b) are conducted under a basic condition The polymer is selected from the group consisting of hyaluronic acid, hyaluronate, carboxymethylcellulous (CMC), alginate, chondroitin-4-sulfate, chondroitin-6-sul fate, Xanthane gum, chitosan, pectin, agar, carrageenan and guar gum thereof and a mixture thereof The method is absent a step of purifying the cross linked hyaluronic acid and the content of the cross-linking agent with free-state functional group is below about 300 ppm in the cross-linked hyaluronic acid Preferably, the method is absent a step of washing the cross-linked hyaluronic acid Preferably, the method is absent a step of dialyzing the cross-linked hyaluronic acid.
4 0042. In describing and claiming the present invention, the following terminologies will be used according to the defini tions set out below The hyaluronate' is a hyaluronic acid composed of a metal ion. Preferably, the hyaluronate is selected from the group consisting of Sodium hyaluronate, potassium hyalur onate and Zinc hyaluronate. 0044) The molecular weight of the polymer selected from the group consisting of hyaluronic acid, hyaluronate, car boxymethylcellulous, alginate, chondroitin-4-sulfate, chon droitin-6-sulfate, Xanthane gum, chitosan, pectin, agar, car rageenan and guar gum thereof and a mixture thereof is not limited to those identified in the invention, and can refer to low molecular weight, high molecular weight, or a mixture having low and high molecular weight of the polymer, such as below 100,000 D; from 100,000 to 500,000 D; from 500,000 to 1,000,000 D; from 1,000,000 to 1,500,000 D; from 1,500, 000 to 2,000,000 D, from 2,500,000 to 3,000,000 D or the like Preferably, a reactant initial concentration of poly mer is from about 2 w/v '% to about 40 w/v '%. More prefer ably, a concentration of polymer is from about 10 w/v '% to about 30 w/v '%. Most preferably, a concentration of polymer is from about 15 w/v '% to about 20 w/v '% The cross-linked hyaluronic acid' has a network structure when the polymer partially cross-linked or thor oughly cross-linked by a cross-linking agent. The cross linked hyaluronic acid may be solid, liquid (when the cross linked hyaluronic acid is dissolved in water or buffer Solution), hydrogel (when the cross-linked hyaluronic acid swells in water or buffer solution), a mixture between liquid and hydrogel or water-insoluble solid. Types of the cross linked hyaluronic acid depend on cross-linked agent content and method used for manufacturing the cross-linked hyalu ronic acid. In the present invention, the cross-linked hyalu ronic acid may be solid, liquid, hydrogel or a mixture of a combination of the above. The solid cross-linked hyaluronic acid may be particle, spongy, Strip, spherical, elliptical or the like In the cross-linking reaction, two of the polymers form a chemical bond such as an ether bond in the present invention between two straight chains without producing by product so a cross-linking reaction can be theoretically pro ceeded completely The method of the invention can be produced under specific conditions (e.g. sterile condition), or common con ditions. Because the method uses a basic condition, bacterium does not grow thereof, therefore avoiding risks of contami nation caused by washing hydrogel Preferably, the temperature in step (a) is from about 35 C. to about 50 C. More preferably, the temperature in step (a) is from about 35 C. to about 40 C. This allows partial cross-linking of the polymer before deterioration begins Preferably, when the temperature in step (a) is from about 35 C. to lower than about 40 C., the cross-linking reaction is proceeded for a period of less than 72 hours; more preferably for 4 to 48 hours; most preferably for 6 to 12 hours Preferably, when the temperature in step (a) is from about 40 C. to lower than about 50 C., the cross-linking reaction is proceeded for a period of less than 48 hours; more preferably for 2 to 24 hours; most preferably for 3 to 6 hours Preferably, when the temperature in step (a) is from about 50 C. to lower than about 60 C., the cross-linking reaction is proceeded for a period of less than 8 hours; more preferably for 0.1 to 2 hours; most preferably proceed for 0.2 to 1 hour Preferably, when the temperature in step (a) is about 60 C., the cross-linking reaction is proceeded for a period of less than 2 hours; more preferably for 0.1 to 0.5 hour; most preferably for 0.2 to 0.3 hour The step (b) according to the invention is lowering the temperature in step (a) to from about 10 C. to about 30 C. for a reaction time of from about 48 hours to about 28 days to obtain the cross-linked hyaluronic acid. Preferably, the temperature in step (b) is from about 10 C. to about 30 C. More preferably, the temperature in step (b) is from about 15 C. to about 30 C. Most preferably, the temperature in step (b) is from about 20 C. to about 30 C. Since hydrolysis of cross-linked hyaluronic acid does not occur under a basic condition at the low temperature, cross-linked hyaluronic acid will not experience rapidly rapidly deterioration and the cross-linking reaction will terminate when the cross-linking agent is consumed to a reasonable concentration Preferably, the reaction time in step (b) is from about 3 days to about 28 days. More preferably, the reaction time in step (b) is from about 3 days to about 11 days. Most prefer ably, the reaction time is from about 3 days to about 7 days. The reaction time is depended on the concentration of the cross-linking agent and base, and the reaction temperature Preferably, the basic condition are prepared by an inorganic base, wherein the inorganic acid is selected from the group consisting of sodium hydroxide, potassium hydrox ide and a mixture thereof Preferably, the basic condition is in a concentration from about 0.05 N to about 1.5 N. More preferably, the concentration is from about 0.05 N to about 1 N. More pref erably, the concentration is from about 0.2N to about 1.5 N. More preferably, the concentration is from about 0.2 N to about 1.0 N. Most preferably, the concentration is from about 0.25 N to about 0.5 N Preferably, the cross-linking agent is a multifunc tional cross-linking agent More preferably, the cross-linking agent is a di functional cross-linking agent Preferably, the cross-linking agent is selected from the group consisting of 1,4-butanediol diglycidyl ether, eth ylene glycol diglycidyl ether, 1.6-hexanediol diglycidyl ether, polypropylene glycol diglycidyl ether, polytetrameth ylene glycol diglycidyl ether, neopentyl glycol diglycidyl ether, polyglycerol polyglycidyl ether, diglycerol polygly cidyl ether, glycerol polyglycidyl ether, trimethylolpropane polyglycidyl ether, pentaerythritol polyglyglycidyl ether, Sor bitol polyglycidyl ether, 1,2,7,8-diepoxyoctane, 1,3-butadi ene diepoxide and a mixture thereof Preferably, a concentration of the cross-linking agent is from about 0.05 w/v '% to about 2 w/v '%. More preferably, a concentration of the cross-linking agent is from about 0.1 w/v % to about 1.5 w/v %. More preferably, a concentration of the cross-linking agent is from about 0.1 W/v % to about 1.0 w/v '%. Most preferably, a concentration of the cross-linking agent is from about 0.6 w/v '% to about 1.0 w/v. % Preferably, the method for producing the cross linked hyaluronic acid further comprises a step of diluting the cross-linked hyaluronic acid. Diluting the cross-linked hyalu ronic acid for adjusting the cross-linked hyaluronic acid to a desired concentration and an acceptable osmotic pressure and
5 ph value in animal physiology may comprise of dilution using water, neutral Solution, buffer Solution, salt Solution or a mixture thereof For example, a preferable concentration of the cross-linked hyaluronic acid is from 5 mg/ml to 60 mg/ml, more preferably from 10 mg/ml to 40 mg/ml, most prefer ably from 20 mg/ml to 30 mg/ml, and a preferable osmotic pressure is from 280 to 340 mosm/kg Preferably, the method for producing the cross linked hyaluronic acid further comprises a step of neutraliz ing cross-linking reaction with ph value preferably between 6.5 and 7.5 so that it can be used on animals. The neutralizing the cross-linking reaction environment step and the diluting the cross-linked hyaluronic acid step may be conducted simultaneously Preferably, the method for producing the cross linked hyaluronic acid further comprises a homogenizing the cross-linked hyaluronic acid step to form a dispersing par ticle The cross-linking agent with a free-state functional group' indicates that the cross-linking agent of at least one function group is un-reacted with the polymer selected from the group consisting of hyaluronic acid, hyaluronate, car boxymethylcellulous, alginate, chondroitin-4-sulfate, chon droitin-6-sulfate, Xanthane gum, chitosan, pectin, agar, car rageenan and guar gum thereof and a mixture thereof Said deterioration in the cross-linked hyaluronic acid can cause the cross-linked hyaluronic acid to turn deep yellow or deep brown after the cross-linking reaction due to hydrolysis of cross-linked hyaluronic acid under a basic con dition, or can cause a final product to degrade obviously. In the present invention, the deterioration is limited to light yellow or light brown just because the cross-linked hyaluric acid used is light yellow or light brown Consuming cross-linking agent within a reasonable concentration indicates that the concentration of the cross linking agent within the cross-linked product can, following the aforementioned steps, be below 1 ppm, 2 ppm, 5 ppm, 10 ppm, 15 ppm, 20 ppm, 100 ppm, 200 ppm, or 300 ppm. However, the consuming concentration of the cross-linking agent is not intended to limit the scope of the present inven tion. The fact that the cross-linking agent is consumed is resulting not only from cross-linking reaction but also from hydrolysis reaction. In an embodiment of the present inven tion, un-reacted cross-linking agent can be controlled at below 1 ppm, 1 ppm to 2 ppm, 2 ppm to 5 ppm, 5 ppm to 10 ppm, 10 ppm to 15 ppm, 15 ppm to 20 ppm, 20 ppm to 100 ppm, 100 ppm to 200 ppm, or 200 ppm to 300 ppm The following examples further illustrate the present invention but are not to be construed as limiting the invention as defined in the claims appended hereto. EXAMPLE Sodium hyaluronate concentration of 20 w/v '%, base concentration of 0.5 N, low reaction temperature of 30 C. and cross-linking agent (1,2,7,8-diepoxyoctane) concen tration of 1 V/v '% have been chosen. ( ml of deionized water is added into 1 ml of 5N sodium hydroxide solution, 0.1 ml of 1,2,7,8-diepoxyoc tane, and 2 g sodium hyaluronate (dry weight) of high molecular weight hyaluronic acid (HHA, average molecular weight 1,350,000), under constant stirring by a magnetic a thermostatic container held at 30 C. for various reaction times as shown in Table The cross-linked hyaluronic acid is added to 79.2 ml of ph of M phosphate buffer solution and 0.8 ml of 6 N hydrogen chloride solution to regulate the ph value and osmotic pressure and to adjust it to be suitable for the physiology of animals; then, the cross-linked hyaluronic A method to determine the amount of cross-linking agent with free-state functional group in the cross-linked hyaluronic acid comprises a hydrolysis step with hyalu ronidase and the method used by Nelis and Sinsheimer (A sensitive Fluorimetric for the Determination of Aliphatic Epoxides under Physiological Conditions, Anal. Biochem. Vol. 115, pp , 1981). A calibration of the above mentioned method provides a good linear model when the content of the cross-linking agent with free-state functional group is between 1 and 300 ppm in the cross-linked hyalu ronic acid g of cross-linked hyaluronic acid is added to a 50 ml certified volumetric flask and 3 ml of 6 N sulfuric acid Solution is added to dissolve the cross-linked hyaluronic acid. After cross-linked hyaluronic acid has dissolved, deionized water is added by a scale of the certified volumetric flask. Then, using the method of European Pharmacopoeia 4.0 (2002) the glucuronic acid content is determined for calcu lating the cross-linked hyaluronic acid content. The cross linked hyaluronic acid content and content of residual cross linking agent with free-state functional group in the product have been recorded with different reaction times as shown in Table 1. EXAMPLE Sodium hyaluronate concentration of 20 w/v '%, base concentration of 0.5 N, low reaction temperature of 30 C. and cross-linking agent (1,3-butadiene diepoxide) concen tration of 1 V/v '% have been chosen The method of example 1 is followed except that the cross-linking agent is now 1,3-butadiene diepoxide. The cross-linked hyaluronic acid content and content of residual cross-linking agent with free-state functional group have been recorded with different reaction times as shown in Table 1. EXAMPLE Sodium hyaluronate concentration of 20 w/v '%, base concentration of 0.25 N. low reaction temperature of 10 C. and cross-linking agent (1,4-butanediol diglycidyl ether) concentration of 1 V/v '% have been chosen ml of deionized water is added to 0.5 ml of 5N Sodium hydroxide Solution, 0.1 ml of 1,4-butanedion digly cidyl ether, and 2 g (dry weight) sodium hyaluronate of high molecular weight hyaluronic acid (HHA, average molecular weight 1,350,000), under constant stirring by a magnetic a thermostatic container maintained at 10 C. for reaction times shown in Table 1. The cross-linked hyaluronic acid is added to 79.6 ml of ph of 0.1 M phosphate buffer
6 solution and 0.4 ml of 6 N hydrogen chloride solution to regulate the ph value and osmotic and to adjust it to be Suitable for the physiology of animals. Then, the cross-linked hyaluronic The method to determine amount of cross-linking agent with free-state functional group in cross-linked hyalu ronic acid in example 1 is used. The cross-linked hyaluronic acid content and content of residual cross-linking agent with free-state functional group have been recorded with different reaction times as shown in Table 1. EXAMPLE Sodium hyaluronate concentration of 20 w/v '%, base concentration of 0.25 N. low reaction temperature of 20 C. and cross-linking agent (1,4-butanediol diglycidyl ether) concentration of 1 V/v '% have been chosen The method of the example 3 is used, except that the low reaction temperature has varied. The cross-linked hyalu ronic acid content and content of residual cross-linking agent with free-state functional group have been recorded with different reaction times as shown in Table 1. EXAMPLE Sodium hyaluronate concentration of 20 w/v '%, base concentration of 0.25 N. low reaction temperature of 30 C. and cross-linking agent (1,4-butanediol diglycidyl ether) concentration of 1 V/v '%have been chosen The method of example3 is used, except that the low reaction temperature has varied. The cross-linked hyaluronic acid content and content of residual cross-linking agent with free-state functional group have been recorded with different reaction times as shown in Table 1. EXAMPLE Sodium hyaluronate concentration of 20 w/v '%, base concentration of 0.2 N, low reaction temperature of 30 C. and cross-linking agent (1,4-butanediol diglycidyl ether) concentration of 1 V/v '% have been chosen ml of deionized water is added to 0.4 ml of 5N Sodium hydroxide Solution, 0.1 ml of 1,4-butanedion digly cidyl ether, and 2 g (dry weight) hyaluronic acid of high molecular weight hyaluronic acid (HHA, average molecular weight 1,350,000), under constant stirring by a magnetic a thermostatic container maintained at 30 C. for reaction times shown in Table 1. The cross-linked hyaluronic acid is added into ml of ph of 0.1 M phosphate buffer solution and 0.32 ml of 6 N hydrogen chloride solution to regulate the ph value and osmotic pressure and to adjust it to be suitable for the physiology of animals. Then, the cross linked hyaluronic The method to determine the amount of the cross linked hyaluronic acid is the same as example 1. The cross linked hyaluronic acid content and the content of residual cross-linking agent with free-state functional group have been recorded with different reaction times as shown in Table Table 1 shows that when the reaction time of the cross-linking reaction is over 2 days, the cross-linked hyalu ronic acid can be used on animals. TABLE 1 Cross-linked agent with Cross-linked Reaction Reaction hyaluronic acid free-state Example temperature ( C.) Time (day) content in product (w/v 96) functional group in product (ppm) S <1% <1% <1% 4 2O <1% <1% OO OS <1% Note: *represents it is not in the range of calibration (between 1 to 300 ppm). EXAMPLE 7 I0088 Sodium hyaluronate concentration of 10 w/v '%, base concentration of 0.05 N, high reaction temperature of 50 C., low reaction temperature of 30 C. and cross-linking agent (1,4-butanediol diglycidyl ether) concentration of 1 V/v % have been chosen ml of deionized water is added to 0.1 ml of 5 N Sodium hydroxide Solution, 0.1 ml of 1,4-butanedion digly cidyl ether, and 1 g (dry weight) sodium hyaluronate of high molecular weight hyaluronic acid, HHA (average molecular weight 1,350,000) under continuous stirring by a magnetic athermostatic container maintained at 50 C. for 7 hours and then at 30 C. for a specific reaction time shown in Table 2. (0090. The cross-linked hyaluronic acid is added to 9.87 ml of deionized water, 20 ml of ph of 0.15 M phosphate buffer solution and 0.13 ml of 6 N hydrogen chloride solution to regulate the ph value and osmotic pres sure and to adjust it to be suitable for the physiology of animals. Then, the cross-linked hyaluronic acid has been homogenized The method to determine amount of cross-linking agent with free-state functional group in cross-linked hyalu ronic acid is the same as example 1. The cross-linked hyalu ronic acid content and the content of residual cross-linking agent with free-state functional group have been recorded with different reaction times as shown in Table 2. EXAMPLE Sodium hyaluronate concentration of 20 w/v '%, base concentration of 0.5 N, high reaction temperature of 40
7 C., low reaction temperature of 25 C. and cross-linking agent (1,4-butanediol diglycidyl ether) concentration of 1 V/v % have been chosen ml of deionized water is added to 1 ml of 5N sodium hydroxidesolution, 0.1 ml of 1,4-butanediol digly cidyl ether, and 2.0 g (dry weight) sodium hyaluronate of high molecular weight hyaluronic acid, HHA (average molecular weight 1,350,000), under continuous stirring by a magnetic a thermostatic container held at 40 C. for 3 hours and the at 25 C. for a specific reaction time shown in Table The cross-linked hyaluronic acid is added to 79.2 ml of ph of M phosphate buffer solution and 0.8 ml of 6 Nhydrogen chloride solution to regulate ph value and osmotic pressure and to adjust it to be suitable for the physiology of animals. Then, the cross-linked hyaluronic The method to determine the amount of cross-link ing agent with free-state functional group in the cross-linked hyaluronic acid is the same as example 1. The cross-linked hyaluronic acid the content and content of residual cross EXAMPLE Sodium hyaluronate concentration of 20 w/v '%, base concentration of 0.5 N, high reaction temperature of 40 C., low reaction temperature of 25 C. and cross-linking agent (1,2,7,8-diepoxyoctane) concentration of 1 V/v % have been chosen The method of example 8 is used, except that the cross-linking agent is now diepoxyoctane. The cross linked hyaluronic acid content and the content of residual cross-linking agent with free-state functional group have been recorded with different reaction times as shown in Table 2. EXAMPLE Sodium hyaluronate concentration of 20 w/v % (10 w/v % HHA and 10 w/v % LHA), base concentration of 0.25 N. high reaction temperature of 40 C., low reaction tempera ture of 10 C. and cross-linking agent (1,4-butanediol digly cidyl ether) concentration of 0.6 V/v% have been chosen ml of deionized water is added to 0.5 ml of 5N sodium hydroxide solution, 0.06 ml of 1,4-butanediol digly cidyl ether, 1 g (dry weight) of high molecular weight sodium hyaluronate, HHA (average molecular weight 1,350,000) and 1 g (dry weight) of low molecular weight Sodium hyalur onate. LHA (average molecular weight 440,000), under con tinuous stirring by a magnetic stirring bar at room tempera ture for 5 minutes and is placed in a thermostatic container held at 40 C. for 4 hours and then at 10 C. for a specific reaction time shown in Table The cross-linked hyaluronic acid is added to 79.6 ml of ph of 0.1 M phosphate buffer solution and 04 ml of6n hydrogen chloride solution to regulate the ph value and osmotic pressure and to adjust it to be suitable for the physiology of animals. Then, the cross-linked hyaluronic 0101 The method to determine the amount of the cross linked hyaluronic acid of example 1 is used. The cross-linked hyaluronic acid content and the content of residual cross EXAMPLE Sodium hyaluronate concentration of 30 w/v '% (15 w/v % HHA and 15 w/v% LHA), base concentration of 0.25 N. high reaction temperature of 40 C., low reaction tempera ture of 25 C. and cross-linking agent (1,4-butanedionl dig lycidyl ether) concentration of 0.6 V/v % have been chosen. ( ml of deionized water is added to 0.5 ml of 5N sodium hydroxide solution, 0.06 ml of 1,4-butanediol digly cidyl ether, 1.5 g (dry weight) of high molecular weight sodium hyaluronate, HHA (average molecular weight 1,350, 000) and 1.5 g (dry weight) of low molecular weight sodium hyaluronate. LHA (average molecular weight 440,000) under continuous stirring by a magnetic stirring bar at room tem perature for 5 minutes and is placed in a thermostatic con tainer held at 40 C. for 4 hours and then at 25 C. for a specific reaction time as shown in Table The cross-linked hyaluronic acid is added to 79.6 ml of ph of 0.10 Mphosphate buffersolution and 0.4 ml of6n hydrogen chloride solution to regulate the ph value and osmotic pressure and to adjust it to be suitable for the physiology of animals. Then, the cross-linked hyaluronic The method to determine the amount of the cross linked hyaluronic acid of example 1 is used. The cross-linked hyaluronic acid content and the content of residual cross EXAMPLE Sodium hyaluronate concentration of 20 w/v '% LHA, base concentration of 0.25 N, high reaction tempera ture of 40 C., low reaction temperature of 25 C. and cross linking agent (1,4-butanediol diglycidyl ether) concentration of 0.6 V/v '% have been chosen The method of the example 11 is used except 2 g (dry weight) of low molecular weight Sodium hyaluronate is hereby used. The cross-linked hyaluronic acid content and the content of residual cross-linking agent with free-state func tional group have been recorded with different reaction times as shown in Table 2. EXAMPLE Sodium hyaluronate concentration of 20 w/v '% (10 w/v % HHA and 10 w/v% LHA), base concentration of 0.25 N. high reaction temperature of 40 C., low reaction tempera ture of 25 C. and cross-linking agent (1,4-butanediol digly cidyl ether) concentration of 0.1 V/v% have been chosen. 0109) 9.49 ml of deionized water is added to 0.5 ml of 5N sodium hydroxide solution, 0.01 ml of 1,4-butanediol digly cidyl ether, 1.0 g of high molecular weight Sodium hyalur onate, HHA (average molecular weight 1,350,000) and 1.0 g of low molecular weight sodium hyaluronate, LHA (average molecular weight 440,000) under continuous stirring by a magnetic stirringbaratroom temperature for 5 minutes and is placed in a thermostatic container held at 40 C. for 4 hours and then at 25 C. for a specific reaction time shown in Table The cross-linked hyaluronic acid is added to 79.6 ml of ph of 0.10 Mphosphate buffer solution and 0
8 4 ml of 6 N hydrogen chloride solution to regulate the ph value and osmotic pressure and to adjust it to be suitable for the physiology of animals. Then, the cross-linked hyaluronic The method to determine the amount of the cross linked hyaluronic acid of example 1 is used. The cross-linked hyaluronic acid content and the content of residual cross EXAMPLE Sodium hyaluronate concentration of 20 w/v '% LHA, base concentration of 0.2N, high reaction temperature of 40 C., low reaction temperature of 30 C. and cross linking agent (1,4-butanediol diglycidyl ether) concentration of 1 V/v '% have been chosen ml of deionized water is added to 0.4 ml of 5N sodium hydroxide solution, 0.1 ml of 1,4-butanediol digly cidyl ether, and 2.0 g (dry weight) of low molecular weight Sodium hyaluronate, LHA (average molecular weight 440, 000), under continuous stirring by a magnetic stirring bar at room temperature for 5 minutes and is placed in a thermo static container held at 40 C. for 4 hours and then at 30 C. for a specific reaction time shown in Table The cross-linked hyaluronic acid is added to ml of ph of 0.10 Mphosphate buffer solution and 0.32 ml of 6 N hydrogen chloride solution to regulate the ph value and osmotic pressure and to adjust it to be suitable for the physiology of animals. Then, the cross-linked hyaluronic 0115 The method to determine the amount of the cross linked hyaluronic acid of example 1 is used. The cross-linked hyaluronic acid content and the content of residual cross EXAMPLE Sodium hyaluronate concentration of 10 w/v '% HHA, carboxymethyl cellulose (sodium salt) of 10 w/v '%, base concentration of 0.3 N, high reaction temperature of 40 C., low reaction temperature of 20 C. and cross-linking agent (1,4-butanediol diglycidyl ether) concentration of 0.6 V/v % have been chosen ml of deionized water is added to 0.6 ml of 5 Nsodium hydroxide solution, 0.4 g sodium chloride, 0.06 ml of 1,4-butanediol diglycidyl ether, 1.0 g (dry weight) of high molecular weight sodium hyaluronate, HHA, and 1.0 g (dry weight) of carboxymethyl cellulose (Sodium salt) under con tinuous stirring by a magnetic stirring bar at room tempera ture for 5 minutes and is placed in a thermostatic container held at 40 C. for 4 hours and then at 20 C. for a specific reaction time shown in Table The cross-linked hyaluronic acid is added to ml of ph of 0.01 M phosphate buffer solution and 0 5 ml of 6 N hydrogen chloride solution to regulate the ph value and osmotic pressure and to adjust it to be suitable for the physiology of animals. Then, the cross-linked hyaluronic 0119 The method to determine the amount of the cross linked hyaluronic acid of example 1 is used, except that the hydrolysis enzyme is now the mixture of hyaluronidase and cellulase (cellucilast 1.5 L, Novo Nordisk Ferment Ltd.). The cross-linked hyaluronic acid content and the content of residual cross-linking agent with free-state functional group have been recorded with different reaction times as shown in Table 2. I0120 When the reaction time of the cross-linking reaction is over 2 days, the cross-linked hyaluronic acid can be used for animal. TABLE 2 High Low Cross- Cross-linked tel- tel- linked hyaluronic agent with perature perature acid content free-state Ex- Time Time in product functional group ample C. (hours) C. (days) (w/v 96) in product (ppm) S O <1% O % S <1% O < O OS < O : <1% O < SO S O <1% O <1% I0121 Even though numerous characteristics and advan tages of the present invention have been set forth in the foregoing description, together with details of the structure and function of the invention, the disclosure is only illustra tive. Changes may be made to the details, especially regard
9 ing the shape, size, and arrangement of parts within the prin ciples of the invention to the full extent as indicated by the broad general meaning of the terms in which the appended claims are expressed. What is claimed is: 1. A method for producing a cross-linked hyaluronic acid comprising: (a) cross-linking at least one polymer at a temperature, from about 35 C. to about 60 C., for a reaction time of from about 0.1 hour to about 72 hours with a cross linking agent, and (b) lowering the temperature in step (a) to from about 10 C. to about 30 C. for a reaction time of from about 48 hours to about 28 days to obtain the cross-linked hyalu ronic acid, wherein the steps (a) and (b) are conducted under a basic condition; the polymer is selected from the group consisting of hyalu ronic acid, hyaluronate, carboxymethylcellulous (CMC), alginate, chondroitin-4-sulfate, chondroitin-6- Sulfate, Xanthane gum, chitosan, pectin, agar, carrag eenan and guar gum thereof and a mixture thereof, and the method is absent a step of purifying the cross-linked hyaluronic acid and the content of the cross-linking agent with free-state functional group is below about 300 ppm in the cross-linked hyaluronic acid. 2. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the method is absent a step of washing the cross-linked hyaluronic acid. 3. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the method is absent a step of dialyzing the cross-linked hyaluronic acid. 4. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the hyaluronate is selected from the group consisting of sodium hyaluronate, potassium hyaluronate and Zinc hyaluronate. 5. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein a concentration of the polymer is from about 2 w/v '% to about 40 w/v '%. 6. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the temperature in Step (a) is from about 35 C. to about 40 C. 7. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the temperature in step (b) is from about 15 C. to about 30 C. 8. The method for producing the cross-linked hyaluronic acid as claimed in claim 7, wherein the temperature in step (b) is from about 20 C. to about 30 C. 9. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the reaction time in step (b) is from about 3 days to about 28 days. 10. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the basic condition is about 0.05 N to about 1.5 N. 11. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the basic condition is about 0.2N to about 1.5 N. 12. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the basic condition is about 0.2N to about 1.0 N. 13. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the basic condition is achieved by an inorganic base. 14. The method for producing the cross-linked hyaluronic acid as claimed in claim 13, wherein the inorganic base is selected from the group consisting of sodium hydroxide, potassium hydroxide and a mixture thereof. 15. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the cross-linking agent is selected from the group consisting of 1,4-butanediol digly cidyl ether, ethylene glycol diglycidyl ether, 1.6-hexanediol diglycidyl ether, polypropylene glycol diglycidyl ether, poly tetramethylene glycol diglycidyl ether, neopentylglycol dig lycidyl ether, polyglycerol polyglycidyl ether, diglycerol polyglycidyl ether, glycerol polyglycidyl ether, trimethylol propane polyglycidyl ether, pentaerythritol polyglyglycidyl ether, Sorbitol polyglycidyl ether, diepoxyoctane, 1,3-butadiene diepoxide and a mixture thereof. 16. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein a concentration of the cross-linking agent is from about 0.05 w/v '% to about 2 w/v. %. 17. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, further comprising a step of diluting the cross-linked hyaluronic acid. 18. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, further comprising a step of neutralizing the cross-linked hyaluronic acid. 19. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, further comprising a step of homogenizing the cross-linked hyaluronic acid. 20. The method for producing the cross-linked hyaluronic acid as claimed in claim 1, wherein the content of the cross linking agent with free-state functional group is below about 20 ppm.
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
(19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
(12) United States Patent (10) Patent No.: US 6,841,523 B1
 USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
(12) United States Patent
 US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
United States Patent (19)
 United States Patent (19) Lachapelle (54 PROCESS FOR PREPARING A PAPER PULP USING CARBON DOXDE AS AN ACDIFYINGAGENT FOR A BLEACHED PULP (75. Inventor: Raymond C. Lachapelle, Kirkland, Canada 73 Assignee:
United States Patent (19) Lachapelle (54 PROCESS FOR PREPARING A PAPER PULP USING CARBON DOXDE AS AN ACDIFYINGAGENT FOR A BLEACHED PULP (75. Inventor: Raymond C. Lachapelle, Kirkland, Canada 73 Assignee:
(12) United States Patent (10) Patent No.: US 7434,929 B2
 US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1. Reynolds et al. (43) Pub. Date: Jan. 15, 2004
 (19) United States US 2004001 0311A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0010311 A1 Reynolds et al. (43) Pub. Date: (54) CUSTOMIZABLE INTEGRATED (21) Appl. No.: 10/196,311 PROSTHETIC
(19) United States US 2004001 0311A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0010311 A1 Reynolds et al. (43) Pub. Date: (54) CUSTOMIZABLE INTEGRATED (21) Appl. No.: 10/196,311 PROSTHETIC
(12) United States Patent (10) Patent No.: US 6,752,627 B2
 USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
III. United States Patent 19 Jordan 5,389,129. Feb. 14, ). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee:
 United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
Ref. 11. (12) Patent Application Publication (10) Pub. No.: US 2012/ A1. (19) United States. Polstein et al. (43) Pub. Date: Jun.
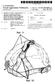 (19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States US 20130244977A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0244977 A1 Lee et al. (43) Pub. Date: (54) COSMETIC BIO-CELLULOSE MASK PACK (30) Foreign Application Priority
(19) United States US 20130244977A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0244977 A1 Lee et al. (43) Pub. Date: (54) COSMETIC BIO-CELLULOSE MASK PACK (30) Foreign Application Priority
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
(19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
(12) United States Patent (10) Patent No.: US 6,308,717 B1
 USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
United States Patent (19)
 United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
United States Patent (19) Frankel
 United States Patent (19) Frankel 11 Patent Number: 45 Date of Patent: Jan. 27, 1987 (54) SWEAT COLLECTING HEADBAND 76) Inventor: Alfred R. Frankel, 403 Gulf Way - Apt. 701, St. Petersburg, Fla. 33706
United States Patent (19) Frankel 11 Patent Number: 45 Date of Patent: Jan. 27, 1987 (54) SWEAT COLLECTING HEADBAND 76) Inventor: Alfred R. Frankel, 403 Gulf Way - Apt. 701, St. Petersburg, Fla. 33706
United States Patent (19) Humbrecht
 United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(12) United States Patent (10) Patent No.: US 6,413,305 B1
 USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
United States Patent (19) Katz
 United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
WWWWW. ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / A1. 19 United States
 THE MAIN TEA ETA AITOR A TT MA N ALUMINIUM TIN US 20170266826A1 19 United States ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / 0266826 A1 Kole et al. ( 43 ) Pub. Date : Sep. 21, 2017
THE MAIN TEA ETA AITOR A TT MA N ALUMINIUM TIN US 20170266826A1 19 United States ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / 0266826 A1 Kole et al. ( 43 ) Pub. Date : Sep. 21, 2017
12) United States Patent 10) Patent No.: US 6,433,024 B1
 USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 2008011 6236A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0116236A1 NICKELS (43) Pub. Date: May 22, 2008 (54) COMBINATION UMBRELLA, SUPPORTAND Publication Classification
(19) United States US 2008011 6236A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0116236A1 NICKELS (43) Pub. Date: May 22, 2008 (54) COMBINATION UMBRELLA, SUPPORTAND Publication Classification
(*) Notice: Slity sight 58 $5 E. G.
 USOO629.7208B1 (12) United States Patent (10) Patent No.: US 6,297,208 B1 Crist () Date of Patent: Oct. 2, 2001 (54) RUST STAIN REMOVAL FORMULA 4,891,0 1/1990 Gross et al.. 4,963,233 * 10/1990 Mathew...
USOO629.7208B1 (12) United States Patent (10) Patent No.: US 6,297,208 B1 Crist () Date of Patent: Oct. 2, 2001 (54) RUST STAIN REMOVAL FORMULA 4,891,0 1/1990 Gross et al.. 4,963,233 * 10/1990 Mathew...
United States Patent (19) Linsten et al.
 United States Patent (19) Linsten et al. 54 PROCESS FOR TREATING OXYGEN DELGN FED PULP USNG AN ORGANIC PERACID OR SALT, COMPLEXINGAGENT AND PEROXIDE BLEACH SEQUENCE 75) Inventors: Magnus Linsten, Kungälv;
United States Patent (19) Linsten et al. 54 PROCESS FOR TREATING OXYGEN DELGN FED PULP USNG AN ORGANIC PERACID OR SALT, COMPLEXINGAGENT AND PEROXIDE BLEACH SEQUENCE 75) Inventors: Magnus Linsten, Kungälv;
(12) United States Patent
 USOO9639 120B2 (12) United States Patent W (10) Patent No.: (45) Date of Patent: May 2, 2017 (54) HEAT DISSIPATIONSTRUCTURE OF WEARABLE ELECTRONIC DEVICE (71) Applicant: ASIA VITAL COMPONENTS CO., LTD.,
USOO9639 120B2 (12) United States Patent W (10) Patent No.: (45) Date of Patent: May 2, 2017 (54) HEAT DISSIPATIONSTRUCTURE OF WEARABLE ELECTRONIC DEVICE (71) Applicant: ASIA VITAL COMPONENTS CO., LTD.,
Hyaluronic acid. and the Advanced. Thixotropic
 HYAcorp_Broschüre_A5_RZ 04.01.16 15:53 Seite 2 Hyaluronic acid Hyaluronic acid (HA) is in use in medicine for a long time with a long safety profile. HA in his natural form has a and the Advanced short
HYAcorp_Broschüre_A5_RZ 04.01.16 15:53 Seite 2 Hyaluronic acid Hyaluronic acid (HA) is in use in medicine for a long time with a long safety profile. HA in his natural form has a and the Advanced short
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
United States Patent (19) Schunter
 United States Patent (19) Schunter 11 45 US005699555A Patent Number: Date of Patent: Dec. 23, 1997 54 CHILD'S WAISTBELTAND LEASH FOR PROTECTIONAGAINSTABDUCTION OF A CHLD 76 Inventor: Christine K. Schunter,
United States Patent (19) Schunter 11 45 US005699555A Patent Number: Date of Patent: Dec. 23, 1997 54 CHILD'S WAISTBELTAND LEASH FOR PROTECTIONAGAINSTABDUCTION OF A CHLD 76 Inventor: Christine K. Schunter,
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(12) United States Patent
 (12) United States Patent Sir et al. USOO68194B2 (10) Patent No.: () Date of Patent: Feb., 2005 (54) INK COMPOSITION CONTAINING MICRO ENCAPSULATED UV ABSORBER AND PROCESS FOR PREPARING THE SAME (75) Inventors:
(12) United States Patent Sir et al. USOO68194B2 (10) Patent No.: () Date of Patent: Feb., 2005 (54) INK COMPOSITION CONTAINING MICRO ENCAPSULATED UV ABSORBER AND PROCESS FOR PREPARING THE SAME (75) Inventors:
The Effects of Shear on Neutralized Carbomers in Aqueous Conditions
 The Effects of Shear on Neutralized Carbomers in Aqueous Conditions Lyndel Speedy 18/07/2014 With thanks to Ensign Laboratories 1 Abstract Carbomer is the generic name for a class of high molecular weight
The Effects of Shear on Neutralized Carbomers in Aqueous Conditions Lyndel Speedy 18/07/2014 With thanks to Ensign Laboratories 1 Abstract Carbomer is the generic name for a class of high molecular weight
United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985
 United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985 54 INK RIBBON CASSETTE PROVIDED WITH 56) References Cited AN EMPREGNATION DEVICE U.S. PATENT DOCUMENTS s 2,76,539
United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985 54 INK RIBBON CASSETTE PROVIDED WITH 56) References Cited AN EMPREGNATION DEVICE U.S. PATENT DOCUMENTS s 2,76,539
ABOUT MD SKIN SOLUTIONS
 Feel the difference ABOUT MD SKIN SOLUTIONS In Luxembourg, MD Skin Solutions develops science-based anti-ageing products, offering strong clinical performance to physicians. A PARTNER YOU CAN TRUST Dedicated
Feel the difference ABOUT MD SKIN SOLUTIONS In Luxembourg, MD Skin Solutions develops science-based anti-ageing products, offering strong clinical performance to physicians. A PARTNER YOU CAN TRUST Dedicated
Hyaluronic acid and the advanced thixotropic
 Made in Germany by Hyaluronic acid and the advanced thixotropic technology (ATT) Hyaluronic acid (HA) has been used in medicine for a long time, and over many years has been proven to have an excellent
Made in Germany by Hyaluronic acid and the advanced thixotropic technology (ATT) Hyaluronic acid (HA) has been used in medicine for a long time, and over many years has been proven to have an excellent
Europaisches Patentamt European Patent Office. Publication number: Office europeen des brevets EUROPEAN PATENT APPLICATION
 J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
Why is pretreatment needed
 Pretreatments Why is pretreatment needed As a whole this process consist of desizing process, scouring and bleaching. Pretreatment process basically aim to removal all impurities found on fiber ( especially
Pretreatments Why is pretreatment needed As a whole this process consist of desizing process, scouring and bleaching. Pretreatment process basically aim to removal all impurities found on fiber ( especially
(12) United States Patent
 (12) United States Patent USOO7374282B2 (10) Patent No.: US 7,374.282 B2 Tendler (45) Date of Patent: May 20, 2008 (54) METHOD AND APPARATUS FOR VIEWING 6,623,116 B2 * 9/2003 Kerns et al.... 351,165 POLARIZED
(12) United States Patent USOO7374282B2 (10) Patent No.: US 7,374.282 B2 Tendler (45) Date of Patent: May 20, 2008 (54) METHOD AND APPARATUS FOR VIEWING 6,623,116 B2 * 9/2003 Kerns et al.... 351,165 POLARIZED
USOO A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999
 USOO5996780A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999 54 COSMETIC APPARATUS 3.513,830 5/1970 Kalayjian... 128/2 3,640,268 2/1972 Davis...... 128/2
USOO5996780A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999 54 COSMETIC APPARATUS 3.513,830 5/1970 Kalayjian... 128/2 3,640,268 2/1972 Davis...... 128/2
(12) United States Patent
 US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
Hydryalix Advantages. Composition. Hydryalix
 2409 Hydryalix Hydryalix (Gentle / Volume / Deep / Ultra Deep / Lips) is an injectable, sterile, apyrogenic gel intended for soft tissue augmentation. It is composed of cross-linked hyaluronic acid from
2409 Hydryalix Hydryalix (Gentle / Volume / Deep / Ultra Deep / Lips) is an injectable, sterile, apyrogenic gel intended for soft tissue augmentation. It is composed of cross-linked hyaluronic acid from
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150343112A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0343112 A1 Dyar et al. (43) Pub. Date: (54) LIQUID BANDAGE Publication Classification (71) Applicant: PROBLEM
(19) United States US 20150343112A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0343112 A1 Dyar et al. (43) Pub. Date: (54) LIQUID BANDAGE Publication Classification (71) Applicant: PROBLEM
(12) United States Patent
 (12) United States Patent Fujiwara USOO6327711B1 (10) Patent No.: (45) Date of Patent: Dec. 11, 2001 (54) STRIP FOR PROVIDING SIMPLIFIED TYPE GARMENTS AND METHOD FOR PROVIDING GARMENTS (75) Inventor: Toshio
(12) United States Patent Fujiwara USOO6327711B1 (10) Patent No.: (45) Date of Patent: Dec. 11, 2001 (54) STRIP FOR PROVIDING SIMPLIFIED TYPE GARMENTS AND METHOD FOR PROVIDING GARMENTS (75) Inventor: Toshio
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 201403,36556A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0336556A1 Pucik (43) Pub. Date: Nov. 13, 2014 (54) POSTURE SUPPORT GARMENT (52) U.S. Cl. CPC... A61F 5/02
(19) United States US 201403,36556A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0336556A1 Pucik (43) Pub. Date: Nov. 13, 2014 (54) POSTURE SUPPORT GARMENT (52) U.S. Cl. CPC... A61F 5/02
(12) United States Patent (10) Patent No.: US 9.407, B2
 USOO9407742B2 (12) United States Patent (10) Patent No.: US 9.407,742 9 9 B2 NOble Nava (45) Date of Patent: Aug. 2, 2016 (54) CELL PHONE HOLSTER 2005/008 (2013.01); A45F 2200/0516 (2013.01); H04B 2001/3861
USOO9407742B2 (12) United States Patent (10) Patent No.: US 9.407,742 9 9 B2 NOble Nava (45) Date of Patent: Aug. 2, 2016 (54) CELL PHONE HOLSTER 2005/008 (2013.01); A45F 2200/0516 (2013.01); H04B 2001/3861
(12) United States Patent (10) Patent No.: US 7,585,200 B1
 US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
(12) United States Patent (10) Patent No.: US 8,108,948 B2
 USOO8108948B2 (12) United States Patent (10) Patent No.: US 8,108,948 B2 B00s (45) Date of Patent: *Feb. 7, 2012 (54) METHOD AND APPARATUS FOR KEEPING A 3,161,932 A 12/1964 Russell SHIRT COLLAR ALIGNED
USOO8108948B2 (12) United States Patent (10) Patent No.: US 8,108,948 B2 B00s (45) Date of Patent: *Feb. 7, 2012 (54) METHOD AND APPARATUS FOR KEEPING A 3,161,932 A 12/1964 Russell SHIRT COLLAR ALIGNED
United States Patent (19)
 United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
United States Patent (19)
 United States Patent (19) Baggetta 54 SAFETY TETHER DEVICE 76 Inventor: Colleen S. Baggetta, 2839 Andiron La., Vienna, Va. 22180 (21) Appl. No.: 901,193 (22 Filed: Aug. 28, 1986 51) Int. Cl."... A62B/00
United States Patent (19) Baggetta 54 SAFETY TETHER DEVICE 76 Inventor: Colleen S. Baggetta, 2839 Andiron La., Vienna, Va. 22180 (21) Appl. No.: 901,193 (22 Filed: Aug. 28, 1986 51) Int. Cl."... A62B/00
(12) (10) Patent No.: US 6,971,424 B1. Angevine (45) Date of Patent: Dec. 6, (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi...
 United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080191205A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0191205 A1 Tsai et al. (43) Pub. Date: Aug. 14, 2008 (54) TEST STRUCTURE FOR SEAL RING (22) Filed: Feb. 13,
(19) United States US 20080191205A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0191205 A1 Tsai et al. (43) Pub. Date: Aug. 14, 2008 (54) TEST STRUCTURE FOR SEAL RING (22) Filed: Feb. 13,
A natural, cost-efficient O/W emulsifier with excellent performance
 Technical Information TEGO Care PSC 3 A natural, cost-efficient O/W emulsifier with excellent performance Intended use O/W emulsifier Benefits at a glance Completely based on natural raw materials Suitable
Technical Information TEGO Care PSC 3 A natural, cost-efficient O/W emulsifier with excellent performance Intended use O/W emulsifier Benefits at a glance Completely based on natural raw materials Suitable
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014009.4718A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0094718A1 Feldman (43) Pub. Date: (54) SYSTEMAND METHOD FORTATTOO (52) U.S. Cl. REMOVAL USPC... 601A2 (71)
(19) United States US 2014009.4718A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0094718A1 Feldman (43) Pub. Date: (54) SYSTEMAND METHOD FORTATTOO (52) U.S. Cl. REMOVAL USPC... 601A2 (71)
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 2016.0143424A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0143424 A1 STEPHENS et al. (43) Pub. Date: May 26, 2016 (54) WEARABLE ELASTIC BAND WITH Publication Classification
(19) United States US 2016.0143424A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0143424 A1 STEPHENS et al. (43) Pub. Date: May 26, 2016 (54) WEARABLE ELASTIC BAND WITH Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0165220 A1 Chang et al. US 201701 65220A1 (43) Pub. Date: (54) (71) (72) (21) (22) (60) (60) TOPCAL PHARMACEUTICAL FORMULATIONS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0165220 A1 Chang et al. US 201701 65220A1 (43) Pub. Date: (54) (71) (72) (21) (22) (60) (60) TOPCAL PHARMACEUTICAL FORMULATIONS
United States Patent (19)
 United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
(19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
United States Patent (19)
 United States Patent (19) Carstens 54 (76) (21) 22) SHIRT FOLDING AND PACKAGING METHOD AND APPARATUS Inventor: Ronald Carstens, 209 Edith Point, Anacortes, Wash. 982 Appl. No.: 188,198 Filed: Apr. 28,
United States Patent (19) Carstens 54 (76) (21) 22) SHIRT FOLDING AND PACKAGING METHOD AND APPARATUS Inventor: Ronald Carstens, 209 Edith Point, Anacortes, Wash. 982 Appl. No.: 188,198 Filed: Apr. 28,
DO DIFFERENT WOUND DRESSINGS PROMOTE WOUND HEALING?
 DO DIFFERENT WOUND DRESSINGS PROMOTE WOUND HEALING? A MUGANZA MD, FCS (SA), FRCSI Head, Burns Unit, Chris Hani Baragwanath Academic Hospital and University of Witwatersrand Wound healing is a complex and
DO DIFFERENT WOUND DRESSINGS PROMOTE WOUND HEALING? A MUGANZA MD, FCS (SA), FRCSI Head, Burns Unit, Chris Hani Baragwanath Academic Hospital and University of Witwatersrand Wound healing is a complex and
III USOO A. 1212,515 l/1917 Leavitt... 5/636 1, /1929 Jonas... 5/ ,000 3/1933 Van Slyck... 5/697
 United States Patent (19) Horowitz 54 76) 21 22 51 52 58 56 ADJUSTABLE BODY SUPPORT WITH MPROVED NECK AND HEAD SUPPORT FILLED WITH GRANULAR MATERAL Inventor: Lawrence Fraser Horowitz, 25 Godwin Ave., Fairlawn,
United States Patent (19) Horowitz 54 76) 21 22 51 52 58 56 ADJUSTABLE BODY SUPPORT WITH MPROVED NECK AND HEAD SUPPORT FILLED WITH GRANULAR MATERAL Inventor: Lawrence Fraser Horowitz, 25 Godwin Ave., Fairlawn,
United States Patent (19)
 USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
AMAZE XT POLYMER (28-029A) Dehydroxanthan Gum
 AMAZE XT POLYMER (28-29A) Dehydroxanthan Gum Performance naturally Today s consumers are educated, driven to succeed and demanding. They want healthy hair that looks good and feels good. Individuality
AMAZE XT POLYMER (28-29A) Dehydroxanthan Gum Performance naturally Today s consumers are educated, driven to succeed and demanding. They want healthy hair that looks good and feels good. Individuality
(12) United States Patent
 (12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
(12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
Int. Cl."... F21V1/06 U.S. C /352; 362/358. References Cited U.S. PATENT DOCUMENTS 3,787,676 l/1974 Korach /352
 United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
thermal Repair Beyond the Bond ProCutiGen Thermal Shield support + protect hair cuticle ProBonding, Keratin derived biomimetic, neo-cuticle
 Code Number: 20828 INCI Name: Hydrolyzed Keratin INCI Status: Conforms REACH Status: Complies CAS Number: 69430-36-0 EINECS Number: 274-001-1. Bivalent Cationic Lipopeptide Repair Beyond the Bond support
Code Number: 20828 INCI Name: Hydrolyzed Keratin INCI Status: Conforms REACH Status: Complies CAS Number: 69430-36-0 EINECS Number: 274-001-1. Bivalent Cationic Lipopeptide Repair Beyond the Bond support
(12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001
 US006257248B1 (12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001 (54) BOTH HAND HAIR CUTTING METHOD 5,991,918 * 11/1999 Choate..... 2/21 6,079,107 * 6/2000
US006257248B1 (12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001 (54) BOTH HAND HAIR CUTTING METHOD 5,991,918 * 11/1999 Choate..... 2/21 6,079,107 * 6/2000
ABIL EM 180 High performance emulsifier for all types of W/O formulations
 ABIL EM 180 High performance emulsifier for all types of W/O formulations Very low usage concentration of down to 0.5 % Excellent stabilization in difficult systems Enhanced performance at high temperatures
ABIL EM 180 High performance emulsifier for all types of W/O formulations Very low usage concentration of down to 0.5 % Excellent stabilization in difficult systems Enhanced performance at high temperatures
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
 US 20100101003A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0101003 A1 McCullough (43) Pub. Date: Apr. 29, 2010 (54) 3 D HELMET WITH CROWN (22) Filed: Oct. 25, 2008 (76)
US 20100101003A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0101003 A1 McCullough (43) Pub. Date: Apr. 29, 2010 (54) 3 D HELMET WITH CROWN (22) Filed: Oct. 25, 2008 (76)
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O18O194A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180194 A1 Cutter (43) Pub. Date: Jul.19, 2012 (54) GARMENTS WITH ADJUSTABLE WAISTS (52) U.S. Cl.... 2/237
(19) United States US 2012O18O194A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180194 A1 Cutter (43) Pub. Date: Jul.19, 2012 (54) GARMENTS WITH ADJUSTABLE WAISTS (52) U.S. Cl.... 2/237
HYDROGEL CATALOGUE. The hydrogel made in France and customizable.
 HYDROGEL CATALOGUE The hydrogel made in France and customizable. PRESENTATION - Hydrogel s properties FORMATS & SIZES - Standard formats 1. Eyes patches 2. Eyes contour masks 3. Face masks 4. Breast and
HYDROGEL CATALOGUE The hydrogel made in France and customizable. PRESENTATION - Hydrogel s properties FORMATS & SIZES - Standard formats 1. Eyes patches 2. Eyes contour masks 3. Face masks 4. Breast and
IIII. United States Patent (19) McCausland. cover removably attached to the outer edge of said
 United States Patent (19) McCausland 54 FACE SHIELD (76) Inventor: Mary L. McCausland, 16629 Lescot Ter. Rockville, Md. 20853 21) Appl. No.: 658,520 22 Filed: Jun. 4, 1996 (51) Int. Cl.... A61F 9/00; A61F
United States Patent (19) McCausland 54 FACE SHIELD (76) Inventor: Mary L. McCausland, 16629 Lescot Ter. Rockville, Md. 20853 21) Appl. No.: 658,520 22 Filed: Jun. 4, 1996 (51) Int. Cl.... A61F 9/00; A61F
United States Patent (19) Steinback
 United States Patent (19) Steinback 54 ELASTIC EXERCISE BANDS AND CUFFS 76 Inventor: Jyl L. Steinback, 15202 N. 50th Pl., Scottsdale, Ariz. 85254 21 Appl. No.: 346,565 22 Filed: Nov. 29, 1994 (51 int.
United States Patent (19) Steinback 54 ELASTIC EXERCISE BANDS AND CUFFS 76 Inventor: Jyl L. Steinback, 15202 N. 50th Pl., Scottsdale, Ariz. 85254 21 Appl. No.: 346,565 22 Filed: Nov. 29, 1994 (51 int.
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 2006004.8272A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0048272 A1 Tison (43) Pub. Date: Mar. 9, 2006 (54) SPORTS HAT (52) U.S. Cl.... 2/175.1 (76) Inventor: Charles
(19) United States US 2006004.8272A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0048272 A1 Tison (43) Pub. Date: Mar. 9, 2006 (54) SPORTS HAT (52) U.S. Cl.... 2/175.1 (76) Inventor: Charles
(12) United States Patent (10) Patent No.: US 9,468,596 B2
 USOO946896B2 (12) United States Patent () Patent No.: Eizen et al. () Date of Patent: Oct. 18, 2016 (4) MULTI USE COSMETIC FORMULA (2013.01); A61K 8/73 (2013.01); A61K 8/737 (2013.01); A61O 1/00 (2013.01);
USOO946896B2 (12) United States Patent () Patent No.: Eizen et al. () Date of Patent: Oct. 18, 2016 (4) MULTI USE COSMETIC FORMULA (2013.01); A61K 8/73 (2013.01); A61K 8/737 (2013.01); A61O 1/00 (2013.01);
REVOLUTIONAL PEPTIDES DERMAL FILLER
 REVOLUTIONAL PEPTIDES DERMAL FILLER Advantages ntages of REVOFIL 1. REVOFIL has both biphasic and monophasic physical characteristics introduced by combination of cutting edged peptide technology and crossed
REVOLUTIONAL PEPTIDES DERMAL FILLER Advantages ntages of REVOFIL 1. REVOFIL has both biphasic and monophasic physical characteristics introduced by combination of cutting edged peptide technology and crossed
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States US 2013 0133.587A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0133587 A1 Pelfrey (43) Pub. Date: May 30, 2013 (54) PET GROOMING AND PEST TERMINATING (52) U.S. Cl. COMB
(19) United States US 2013 0133.587A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0133587 A1 Pelfrey (43) Pub. Date: May 30, 2013 (54) PET GROOMING AND PEST TERMINATING (52) U.S. Cl. COMB
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O130248A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0130248A1 MOZZOne et al. (43) Pub. Date: (54) TOPICAL ANTI-INFLAMMATORY COMPOSITIONS (76) Inventors: Keith
(19) United States US 2003O130248A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0130248A1 MOZZOne et al. (43) Pub. Date: (54) TOPICAL ANTI-INFLAMMATORY COMPOSITIONS (76) Inventors: Keith
TEPZZ 67Z447B_T EP B1 (19) (11) EP B1 (12) EUROPEAN PATENT SPECIFICATION
 (19) TEPZZ 67Z447B_T (11) EP 2 670 447 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 01.07. Bulletin /27 (21) Application number: 1270236.9 (22)
(19) TEPZZ 67Z447B_T (11) EP 2 670 447 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 01.07. Bulletin /27 (21) Application number: 1270236.9 (22)
United States Patent (19)
 United States Patent (19) Long (54) LONG-SLEEVED GARMENT WITH NTEGRATED ANMALDESIGN AND PUPPET-LIKE SLEEVE 76) Inventor: Marla M. Long, 1486 McTaggart Rd., Stow, Ohio 44224 21 Appl. No.: 351,210 (22 Filed:
United States Patent (19) Long (54) LONG-SLEEVED GARMENT WITH NTEGRATED ANMALDESIGN AND PUPPET-LIKE SLEEVE 76) Inventor: Marla M. Long, 1486 McTaggart Rd., Stow, Ohio 44224 21 Appl. No.: 351,210 (22 Filed:
TAGRAVIT TM R1 Encapsulated pure retinol. March 2015
 TAGRAVIT TM R1 Encapsulated pure retinol March 2015 Retinol- Background Retinol is a form of Vitamin A having a small molecular structure with MW of 286.45 g/mol. Retinol can penetrate the outer layers
TAGRAVIT TM R1 Encapsulated pure retinol March 2015 Retinol- Background Retinol is a form of Vitamin A having a small molecular structure with MW of 286.45 g/mol. Retinol can penetrate the outer layers
(12) United States Patent
 (12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
(12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
(12) United States Patent (10) Patent No.: US 6,894,087 B2
 USOO68987B2 (12) United States Patent (10) Patent No.: Batlaw () Date of Patent: May 17, 2005 (54) TONER COMPOSITIONS FOR BLACK 4,284,729 A 8/1981 Cross et al.... 521/8 GRAVURE INKS 4,383,8 A 5/1983 Iyengar......
USOO68987B2 (12) United States Patent (10) Patent No.: Batlaw () Date of Patent: May 17, 2005 (54) TONER COMPOSITIONS FOR BLACK 4,284,729 A 8/1981 Cross et al.... 521/8 GRAVURE INKS 4,383,8 A 5/1983 Iyengar......
organiq FAMILY INTELLIGENT SOLUTIONS
 organiq FAMILY INTELLIGENT SOLUTIONS Content organiq... 2 organiq BLEACH... 3 Spray application... 5 Fog application... 7 organiq BIOPOWER... 10 organiq NEUTRAL... 13 Finishes... 15 1 organiq Family stands
organiq FAMILY INTELLIGENT SOLUTIONS Content organiq... 2 organiq BLEACH... 3 Spray application... 5 Fog application... 7 organiq BIOPOWER... 10 organiq NEUTRAL... 13 Finishes... 15 1 organiq Family stands
(12) United States Patent (10) Patent No.: US 6,620,158 B2
 USOO6620158B2 (12) United States Patent (10) Patent No.: Ronci (45) Date of Patent: Sep. 16, 2003 (54) METHOD OF HAIR REMOVAL 5,868,738 A * 2/1999 Mehl, Sr.... 606/36 6,063,076 A * 5/2000 Mehl et al....
USOO6620158B2 (12) United States Patent (10) Patent No.: Ronci (45) Date of Patent: Sep. 16, 2003 (54) METHOD OF HAIR REMOVAL 5,868,738 A * 2/1999 Mehl, Sr.... 606/36 6,063,076 A * 5/2000 Mehl et al....
FORMATION OF NOVEL COMPOSITE FIBRES EXHIBITING THERMOCHROMIC BEHAVIOUR
 FORMATION OF NOVEL COMPOSITE FIBRES EXHIBITING THERMOCHROMIC BEHAVIOUR L. van der Werff 1,2,3 *, I. L. Kyratzis 1, A. Robinson 2, R. Cranston 1, G. Peeters 1 1 CSIRO Materials Science and Engineering,
FORMATION OF NOVEL COMPOSITE FIBRES EXHIBITING THERMOCHROMIC BEHAVIOUR L. van der Werff 1,2,3 *, I. L. Kyratzis 1, A. Robinson 2, R. Cranston 1, G. Peeters 1 1 CSIRO Materials Science and Engineering,
(12) United States Patent (10) Patent No.: US 7.427,133 B2
 USOO7427133B2 (12) United States Patent (10) Patent No.: US 7.427,133 B2 Carter (45) Date of Patent: Sep. 23, 2008 (54) EARPIECE-LESS EYEGLASS FRAME 5.313,671 A * 5/1994 Flory... 2,428 HAVING AREMOVABLE
USOO7427133B2 (12) United States Patent (10) Patent No.: US 7.427,133 B2 Carter (45) Date of Patent: Sep. 23, 2008 (54) EARPIECE-LESS EYEGLASS FRAME 5.313,671 A * 5/1994 Flory... 2,428 HAVING AREMOVABLE
Tomura, Kazuyo Osaka-shi, Osaka-fu (JP)
 * OJII Eur Pean Patent Office mi mi ii mi 111 ii mi 111 iii Office eurodeen des brevets (11) E P 0 7 1 6 8 4 6 A 1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51 ) nt. CI.6: A61 K 7/1 3
* OJII Eur Pean Patent Office mi mi ii mi 111 ii mi 111 iii Office eurodeen des brevets (11) E P 0 7 1 6 8 4 6 A 1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51 ) nt. CI.6: A61 K 7/1 3
Characteristic of hydrophobically-modified hydroxypropyl methylcellulose, and application of hair cosmetics.
 Characteristic of hydrophobically-modified hydroxypropyl methylcellulose, and application of hair cosmetics. Abstract : Hydrophobically-modified hydroxypropyl methylcellulose (HM-HPMC) is a thickener which
Characteristic of hydrophobically-modified hydroxypropyl methylcellulose, and application of hair cosmetics. Abstract : Hydrophobically-modified hydroxypropyl methylcellulose (HM-HPMC) is a thickener which
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 20060231567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0231567 A1 Perrone (43) Pub. Date: Oct. 19, 2006 (54) CON OPERATED SUNTAN LOTION SPRAY Publication Classification
US 20060231567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0231567 A1 Perrone (43) Pub. Date: Oct. 19, 2006 (54) CON OPERATED SUNTAN LOTION SPRAY Publication Classification
(12) United States Patent (10) Patent No.: US 6,422,036 B1. Giannis et al. (45) Date of Patent: Jul. 23, 2002
 USOO6422036B1 (12) United States Patent (10) Patent No.: US 6,422,036 B1 Giannis et al. (45) Date of Patent: Jul. 23, 2002 (54) JEWELRY CLASP 4,611,368 9/1986 Battersby... 24/116 R 5,214,940 A * 6/1993
USOO6422036B1 (12) United States Patent (10) Patent No.: US 6,422,036 B1 Giannis et al. (45) Date of Patent: Jul. 23, 2002 (54) JEWELRY CLASP 4,611,368 9/1986 Battersby... 24/116 R 5,214,940 A * 6/1993
Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries.
 Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. I. Preservation Naturally-derived product Broad spectrum protection Globally accepted II. Moisturization
Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. I. Preservation Naturally-derived product Broad spectrum protection Globally accepted II. Moisturization
Annex A. Composition of laundry detergents
 Annex A Composition of laundry detergents Laundry detergents are composed of a range of ingredients that give the final product its different characteristics. The most common ingredients are listed alphabetically
Annex A Composition of laundry detergents Laundry detergents are composed of a range of ingredients that give the final product its different characteristics. The most common ingredients are listed alphabetically
(12) United States Patent (10) Patent No.: US 9, B2
 USOO9566228B2 (12) United States Patent (10) Patent No.: US 9,566.228 B2 Jeong (45) Date of Patent: Feb. 14, 2017 (54) BUBBLE TYPE WATERLESS SHAMPOO (2013.01); A61K 8/442 (2013.01); A61K 8/64 COMPOSITION
USOO9566228B2 (12) United States Patent (10) Patent No.: US 9,566.228 B2 Jeong (45) Date of Patent: Feb. 14, 2017 (54) BUBBLE TYPE WATERLESS SHAMPOO (2013.01); A61K 8/442 (2013.01); A61K 8/64 COMPOSITION
Hyalurosmooth. by Beauty Creations. Natural fine line and wrinkle filler
 Hyalurosmooth by Beauty Creations Natural fine line and wrinkle filler Hyalurosmooth Botanical alternative to hyaluronic acid Smoothing and filling of fine lines and wrinkles by injecting «fillers» such
Hyalurosmooth by Beauty Creations Natural fine line and wrinkle filler Hyalurosmooth Botanical alternative to hyaluronic acid Smoothing and filling of fine lines and wrinkles by injecting «fillers» such
Product Information Gluconolactone and Sodium Benzoate (GSB)
 The Soap Kitchen Unit 8 Caddsdown Industrial Park, Clovelly Road, Bideford, Devon EX39 3DX Tel: 01237 420872 (+44 (0)1237 420872) Email: info@thesoapkitchen.co.uk Product Information Gluconolactone and
The Soap Kitchen Unit 8 Caddsdown Industrial Park, Clovelly Road, Bideford, Devon EX39 3DX Tel: 01237 420872 (+44 (0)1237 420872) Email: info@thesoapkitchen.co.uk Product Information Gluconolactone and
Facial Fillers Dr Tarek Said Professor of Plastic Surgery Cairo University 2010
 Facial Fillers Dr Tarek Said Professor of Plastic Surgery Cairo University 2010 Synthetic Fillers (HA fillers, Non-HA fillers including Collagen) Autologous Fat Facial Implantables (AlloDerm & Gore-Tex)
Facial Fillers Dr Tarek Said Professor of Plastic Surgery Cairo University 2010 Synthetic Fillers (HA fillers, Non-HA fillers including Collagen) Autologous Fat Facial Implantables (AlloDerm & Gore-Tex)
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 2011 0041247A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0041247 A1 M00n (43) Pub. Date: Feb. 24, 2011 (54) ALLERGEN-BARRIER BEDDING COVER (52) U.S. Cl.... 5/490;
(19) United States US 2011 0041247A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0041247 A1 M00n (43) Pub. Date: Feb. 24, 2011 (54) ALLERGEN-BARRIER BEDDING COVER (52) U.S. Cl.... 5/490;
CHEMICAL HAIR RELAXERS
 CHEMICAL HAIR RELAXERS CHEMICAL HAIR RELAXERS CHEMICAL HAIR RELAXING IS THE PROCESS OF REARRANGING THE BASIC STRUCTURE OF EXTREMELY CURLY HAIR INTO A STRAIGHT OR LESS CURLY FORM. THE CHEMICAL PROCESS IS
CHEMICAL HAIR RELAXERS CHEMICAL HAIR RELAXERS CHEMICAL HAIR RELAXING IS THE PROCESS OF REARRANGING THE BASIC STRUCTURE OF EXTREMELY CURLY HAIR INTO A STRAIGHT OR LESS CURLY FORM. THE CHEMICAL PROCESS IS
(12) United States Patent (10) Patent No.: US 8,770,209 B2
 US008770209B2 (12) United States Patent (10) Patent No.: Kim et al. (45) Date of Patent: Jul. 8, 2014 (54) COLOR HIGHILIGHTING COSMETICS (52) U.S. Cl. CONTAINER INCLUDING ADETACHABLE USPC... 132/297: 132/318;
US008770209B2 (12) United States Patent (10) Patent No.: Kim et al. (45) Date of Patent: Jul. 8, 2014 (54) COLOR HIGHILIGHTING COSMETICS (52) U.S. Cl. CONTAINER INCLUDING ADETACHABLE USPC... 132/297: 132/318;
