Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
|
|
|
- Garry Malcolm McLaughlin
- 5 years ago
- Views:
Transcription
1 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18
2 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS... 4 A. Risk Assessment... 4 B. Biohazard Spill Kit... 5 V. BIOHAZARDOUS SPILL EMERGENCY RESPONSE... 5 A. Biohazardous Spill Inside a Biological Safety Cabinet (BSC)... 5 i. Spill inside a BSC that stays contained on the work surface... 5 ii. Spill inside a BSC that flows past the work surface through the front or rear grills. 6 B. Biohazardous Spill Outside a Biological Safety Cabinet (BSC)... 7 i. Small spills that can be easily absorbed by one paper towel... 7 ii. Large spills that require more than one paper towel to absorb... 7 iii. Spills inside of a centrifuge... 8 C. Spills Outside the Laboratory in Public Spaces... 9 VI. EXPOSURE RESPONSE AND REPORTING PROCEDURES... 9 A. Personnel Exposure Procedures... 9 B. Medical Treatment... 9 C. Reporting Exposures, Incidents, Accidental Releases VII. EXPOSURE CONTROL PROCEDURES FOR BSL2 RESEARCH A. Overview B. Engineering Hazard Controls C. Work Practice Controls D. Personal Protective Equipment E. Posting Hazard Requirements EHS Office: Phone: (603) ehs@dartmouth.edu Written by: B. Petrella Page: 2 of 13 Approved by: IBC Approval date: 10/3/18
3 I. PURPOSE To establish emergency response and cleanup procedures for laboratory spills involving biohazards in Biosafety Level 1 (BSL1) and Biosafety Level 2/2+ (BSL2/2+) laboratories at in accordance with: CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL) NIH Guidelines for Research Involving Recombinant or Synthetic DNA Molecules (NIH Guidelines) OSHA Bloodborne Pathogens Standard, 29 CFR In order to comply with federal reporting requirements and to ensure timely and appropriate follow-up, Principal Investigators shall immediately report exposures and releases involving recombinant or synthetic nucleic acid (r/sna) molecules as well as violations of the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines, 2013) to the Biological Safety Officer (BSO). This document outlines incident reporting procedures. II. DEFINITIONS For the purposes of this Emergency Response and Exposure Control Plan, biohazards are defined as any material or agent that may contain infectious or potentially infectious substances, or any agents or substances that are an environmental release risk (i.e., recombinant DNA). Examples: Microbiological cultures or stocks (including bacterial, viral, parasitic, fungal, etc.) Recombinant or synthetic nucleic acid molecules (including viral vectors) Organisms or cells that contain recombinant or synthetic nucleic acid molecules (including transgenic organisms and those transiently containing exogenous nucleic acids) Human or animal cell or tissue cultures Anatomical or pathological waste (human or animal tissue or organs) Human clinical specimens (feces, blood, urine or any other bodily fluid) According to the NIH Guidelines (Section I-B), recombinant or synthetic nucleic acids are defined as: molecules that a) are constructed by joining nucleic acid molecules and b) that can replicate in a living cell, i.e., recombinant nucleic acids; nucleic acid molecules that are chemically or by other means synthesized or amplified, including those that are chemically or otherwise modified but can base pair with naturally occurring nucleic acid molecules, i.e., synthetic nucleic acids, or molecules that result from the replication of those described in (i) or (ii) above. Written by: B. Petrella Page: 3 of 13 Approved by: IBC Approval date: 10/3/18
4 III. RESPONSIBILTIES Principal Investigators (PIs) and research laboratory staff who conduct biological or biomedical research or who work in biological or biomedical teaching laboratories must abide by the guidelines outlined in this Plan. It is the responsibility of the PI and laboratory supervisor to ensure that: i. An appropriate spill response plan has been developed for their lab using these generic guidelines as a basis, ii. A copy of this Plan and/or lab specific plans are available to lab staff, iii. Compliance is maintained, iv. Appropriate disinfectants, personal protective equipment, and waste containers are readily available, and v. Reporting and follow-up procedures as described herein are conducted for all incidents. The Dartmouth Environmental Health & Safety (EHS) Office provides OSHAmandated bloodborne pathogen training and biosafety training to all laboratory staff with potential exposures. It is the responsibility of each supervisor to ensure all personnel under his/her supervision complete this required training. The PI will provide EHS with updated emergency contact info when applicable to ensure laboratory door signage is up to date. IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS A. Risk Assessment When assessing a spill to determine the appropriate response, the following should be considered: 1. What was spilled or released? a. Liquid, solid, animal, etc. b. What is the pathogenicity or route of transmission? Consult the BMBL and/or the Canadian Pathogen Safety Data Sheets for guidance. 2. How much was spilled? What is the volume and concentration of the organism? 3. Where is the spill? Is it in a biosafety cabinet, in the lab, outside the lab, etc.? 4. What is the potential for release outside of the lab? Written by: B. Petrella Page: 4 of 13 Approved by: IBC Approval date: 10/3/18
5 B. Biohazard Spill Kit The following items should be assembled in a single container for easy transport to the spill area. Spill kits must be available at all times. A large bucket is a practical container as it may double as the secondary container for waste removal following cleanup. Kit contents: An appropriate chemical decontaminant: In most cases a 1:10 dilution of freshly prepared household bleach is appropriate. Materials to absorb liquids after decontamination: This may include paper towels, absorbent pads, or other materials designed to absorb large volumes of liquid. Appropriate PPE to wear during cleanup: Nitrile or heavy duty gloves, a long-sleeved laboratory coat or gown, and goggles are always necessary. Mucous membrane protection should be considered for large spills. A mechanical means for handling broken glass: This may include tongs, forceps, small disposable scoops and sponges, autoclavable dustpans, or any other method that prevents direct contact with the broken glass. Biohazard bags, sharps containers, and/or other containers: The containers are used to hold the material for further treatment and disposal. V. BIOHAZARDOUS SPILL EMERGENCY RESPONSE A. Biohazardous Spill Inside a Biological Safety Cabinet (BSC) This section provides spill cleanup procedures for biohazardous agents, including recombinant or synthetic nucleic acids, inside a BSC. i. Spill inside a BSC that stays contained on the work surface: 1. Operate the BSC to prevent escape of contaminants from the cabinet. Spill cleanup procedures should be initiated immediately while the cabinet continues to run. 2. Remove contaminated sharps from the spill area using mechanical means (e.g. tongs or forceps). Never remove contaminated sharps by hand. Discard contaminated sharps in a sturdy, leak-proof, biohazard labeled sharps container. 3. Cover the spill with paper towels or other absorbent material. Slowly pour an agent-appropriate disinfectant such that the solution flows into the spill. Paper towels soaked with the disinfectant may also be used to cover the area. A freshly prepared 1:10 dilution of household bleach used with a 20 minute contact time, and followed by a water rinse to prevent corrosion is sufficient in most cases. However, refer to the laboratory specific disinfection protocol for the appropriate disinfectant to use. 4. A minimum of 20 minutes is generally considered an appropriate contact time for thorough decontamination, but the length of time depends on Written by: B. Petrella Page: 5 of 13 Approved by: IBC Approval date: 10/3/18
6 disinfectant and biohazard. Follow manufacturer s recommendations and laboratory specific disinfection protocol for the given biohazard. 5. Wipe up the spill, work surfaces, walls, and any equipment in the cabinet with paper towels dampened with decontaminant. Do not place your head in the cabinet to clean the spill; keep your face behind the sash. 6. Place contaminated paper towels and other spill cleanup materials in orange autoclave biohazard bags. 7. Decontaminate the spill area a second time, placing all used spill materials into a biohazard bag. 8. Remove any contaminated PPE in a manner to avoid crosscontamination; dispose of per standard lab practices. 9. Wash hands thoroughly after removing gloves and other PPE. ii. Spill inside a BSC that flows past the work surface through the front or rear grills: A large spill inside a BSC that flows past the work surface through the front or rear grills requires more extensive decontamination. To prevent escape of contaminants from the cabinet, spill cleanup procedures should be initiated immediately while the cabinet continues to run. 1. Ensure the drain valve under the BSC is closed. 2. Remove contaminated sharps from the spill area using mechanical means (e.g. tongs or forceps). Never remove contaminated sharps by hand. Discard contaminated sharps in a sturdy, leak-proof, biohazard labeled sharps container. 3. Flood the top work surface tray and, if a Class II BSC, the drain pans and catch basins below the work surface with an agent appropriate disinfectant solution. A freshly prepared 1:10 dilution of household bleach used with a 20 minute contact time, and followed by a water rinse to prevent corrosion is sufficient in most cases. However, refer to the laboratory specific disinfection protocol for the appropriate disinfectant to use. 4. A minimum of 20 minutes is generally considered an appropriate contact time for thorough decontamination, but the length of time depends on disinfectant and biohazard. Follow manufacturer s recommendations and laboratory specific disinfection protocol for the given biohazard. 5. Remove excess decontaminant from the work surface tray by wiping with a sponge or cloth. For Class II BSCs, drain the tray into the catch basin below the work surface, lift the tray and take out the removable front intake grille. Wipe the top and bottom (underside) surfaces of the grille with a sponge or cloth soaked in the decontaminant. Then place the tray in position, drain the decontaminant from the cabinet base into an appropriate container, and dispose of the decontaminant in the sewer. 6. Place spill cleanup materials (e.g. contaminated gloves, cloth and/or sponge) in autoclavable pans with lids for autoclaving. 7. Decontaminate the spill area again. Place all used spill materials into a biohazard bag. 8. Remove any contaminated PPE in a manner to avoid crosscontamination and dispose of per standard lab practices. 9. Wash hands thoroughly after removing gloves and other PPE. Written by: B. Petrella Page: 6 of 13 Approved by: IBC Approval date: 10/3/18
7 B. Biohazardous Spill Outside a Biological Safety Cabinet (BSC) This section provides spill cleanup procedures for biohazardous agents, including recombinant or synthetic nucleic acids, inside a BSC. i. Small spills that can be easily absorbed by one paper towel a. Agents transmitted by inhalation (e.g., adenovirus, influenza) 1. Warn other lab members, evacuate the room and close the door to allow aerosols to settle (about 30 minutes). 2. Post a sign on the door and prevent others from entering the contaminated area. 3. Remove contaminated garments and put into a container for autoclaving. Thoroughly wash any exposed areas of the body. 4. Wait 30 minutes to allow aerosols to dissipate. 5. Proceed to ii-4 below. b. Agents NOT transmitted by inhalation 1. Assemble spill cleanup materials. 2. Put on appropriate PPE (e.g. long-sleeved lab coat, goggles, and nitrile gloves). 3. Remove contaminated sharps from the spill area using mechanical means (e.g. tongs or forceps). Discard contaminated sharps in a sturdy, leak-proof, biohazard labeled sharps container. 4. Pour a disinfectant solution (e.g. freshly prepared 1:10 dilution of household bleach) around the spill and allow it to flow into the spill. Paper towels soaked with the decontaminant may also be used to cover the area. To avoid aerosolization, never pour decontaminant solution directly onto the spill. Refer to the laboratory specific disinfection protocol for the appropriate disinfectant to use. 5. Let stand for 20 minutes to allow an adequate contact time and place all used spill materials in a biohazard bag. The length of time depends on disinfectant and biohazard. Follow manufacturer s recommendations and laboratory specific disinfection protocol for the given biohazard. 6. Decontaminate the spill area a second time. Place all used spill materials into a biohazard bag. 7. Remove any contaminated PPE in a manner to avoid cross contamination and dispose of per standard lab practices. 8. Wash hands thoroughly after removing gloves and other PPE. ii. Large spills that require more than one paper towel to absorb 1. Evacuate the room immediately and warn other lab occupants of the spill. Close the door and wait 30 minutes to allow aerosols to dissipate. 2. Post a sign on the door and prevent others from entering the contaminated area. 3. Remove contaminated garments and put into a container for autoclaving. Thoroughly wash any exposed areas of the body. 4. Assemble spill cleanup materials. Written by: B. Petrella Page: 7 of 13 Approved by: IBC Approval date: 10/3/18
8 5. Put on appropriate PPE (e.g. long-sleeved gown, goggles, and nitrile or heavy duty gloves) before re-entering the room. 6. Remove contaminated sharps from the spill area using mechanical means (e.g. tongs or forceps). Discard contaminated sharps in a sturdy, leak-proof, biohazard labeled sharps container. 7. Pour a disinfectant solution (e.g. a freshly prepared 1:10 dilution of household bleach) around the spill and allow it to flow into the spill. Paper towels soaked with the decontaminant may be used to cover the area. To avoid aerosolization, never pour decontaminant solution directly onto the spill. Refer to the laboratory specific disinfection protocol for the appropriate disinfectant to use. 8. A minimum of 20 minutes is generally considered an appropriate contact time for thorough decontamination, but the length of time depends on disinfectant and biohazard. Follow manufacturer s recommendations and laboratory specific disinfection protocol for the given biohazard. 9. Using an autoclavable dustpan and squeegee, transfer contaminated materials (e.g. paper towels, glass, gloves, etc.) to an autoclave bag. 10. Decontaminate the spill area a second time, placing all used spill materials into autoclave pan. 11. Separate reusable items from non-autoclavable plastic as the plastic will melt. Cover the pan with a lid and autoclave according to standard directions. 12. Remove any contaminated PPE in a manner to avoid crosscontamination and dispose of per standard lab practices. 13. Wash hands thoroughly after removing gloves and other PPE. iii. Spills inside of a centrifuge Spills or breakage inside of an operating centrifuge pose a serious potential for exposure due to the creation of aerosols. If a primary container has broken in a centrifuge without a closed rotor or bucket, immediately suspend use, notify lab staff and PI and request assistance from the Biosafety Officer. For suspected or confirmed spills/breakage in any centrifuge, wait at least 30 minutes after the centrifuge has stopped operating to initiate cleanup. 1. Put on lab coat, gloves and a face shield prior to opening centrifuge. Open carefully to assess the damage. 2. If the spill is contained within a closed cup, bucket or rotor, spray the exterior with disinfectant and allow 20 minutes of contact time. Transfer the carrier to the nearest biosafety cabinet (BSC). If a biosafety cabinet is not available, close the centrifuge and post a sign warning of the spill. Notify the PI and BSO for assistance. 3. If a BSC is available, gather supplies needed, such as a sharps container for broken glass and bins filled with disinfectant and place into the BSC. Use forceps to remove broken glass and place directly into a sharps container. Carefully remove any unbroken tubes and place into a bin filled with disinfectant for 20 minutes. Wipe carrier with disinfectant. Written by: B. Petrella Page: 8 of 13 Approved by: IBC Approval date: 10/3/18
9 4. After disinfection, the carrier, bucket or rotor should be washed with a mild soap and water. 5. Spray the interior of the centrifuge chamber with a disinfectant, let sit for 20 minutes and then wipe down. 6. Dispose of all cleanup materials (except sharps) in orange autoclave bags. Dispose of sharps in biohazard sharps containers. 7. Remove protective clothing and thoroughly wash hands. For large centrifuge spills or failures, contact EHS (603) C. Spills Outside the Laboratory in Public Spaces Samples must be transported in secondary, leak-proof containers to minimize the potential for spills. However, if a spill does occur in a common hallway or public space and cannot be immediately decontaminated, cordon off the area, restrict access, and contact EHS immediately at (603) for consultation. VI. EXPOSURE RESPONSE AND REPORTING PROCEDURES A. Personnel Exposure Procedures i. Exposures: Needlesticks or other percutaneous injuries from a contaminated sharp. Splashes to mucous membranes (e.g. eyes, nose, mouth). Bites/scratches from animals that have been exposed to any recombinant or synthetic nucleic acid material, whether or not the exposure leads to illness. ii. Immediate Response: SKIN exposure: Immediately remove contaminated personal protective equipment or clothing and wash the contaminated area with an iodine solution or antibacterial soap and copious water for 10 minutes. EYE exposure: Flush the eye with water for at least 15 minutes at an eyewash station. iii. Notify PI or supervisor. If PI/supervisor is not available, immediately proceed to next step. B. Medical Treatment During work hours, report to Dick s House (Hanover campus) between 7:30am-4:30pm (603) if you are a student -OR- Report to Occupational Medicine (DHMC) between 7:30am-4:30pm (603) ; DHMC, Faulkner Building, Level 4 (near parking garage) if you are an employee Bring along Safety Data Sheets or other literature pertaining to any chemical or biohazard exposure After hours and weekends: report to DHMC Emergency Room. If transport assistance is needed, contact Safety and Security at (603) (or 5555 if at DHMC). If exposure requires emergency treatment, call 911. Written by: B. Petrella Page: 9 of 13 Approved by: IBC Approval date: 10/3/18
10 C. Reporting Exposures, Incidents, Accidental Releases i. Notify the Biological Safety Officer (BSO) immediately. ii. The BSO will investigate the incident and notify the IBC Chair and EHS Director. iii. The PI will complete an internal Incident Report Form and submit it to the BSO and Risk Management within 24 hours. iv. If the IBC Chair and BSO determine that the incident involves non-exempt r/sna molecules, the BSO will submit an NIH incident report to the NIH Office of Biotechnology Activities within 30 days. Incidents occurring in BSL2 laboratories resulting in an overt exposure will be immediately reported to NIH OBA. v. The Office of Risk Management and the Vice Provost for Research will also receive a copy of the incident report. VII. EXPOSURE CONTROL PROCEDURES FOR BSL2/BSL2+ RESEARCH A. Overview Improved engineering and regulation of work practices are the primary means of elimination or minimization of exposure for personnel. Where the possibility of occupational exposure remains, personal protective equipment is to be used. Universal Precautions are used to prevent exposure to bodily fluids/tissues or other materials containing biological pathogens. These precautions are to be taken at all times when working with all BSL2 agents or when the risk of exposure/contamination is present. All clinical specimens of blood, human tissue and bodily fluids are to be handled utilizing BSL2 work practices and procedures. These practices, procedures and facility requirements are described in detail in the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL, 5th edition) and are to be followed by all laboratories working with biological materials. B. Engineering Hazard Controls Engineering controls are to be utilized in circumstances in which an occupational exposure exists. Primary Barriers. Class II Type A Biological Safety Cabinets (BSC) or other physical containment devices must be used when aerosol-creating procedures are performed. Such procedures include centrifuging, grinding, vortexing, blending, sonication and opening containers of infectious materials. Intranasal inoculations or other animal procedures that have the potential for producing splashes and aerosols must be performed in a biological safety cabinet. In special cases, procedures (such as an animal necropsy) may be performed on an open bench if it is determined by the Biological Safety Officer that the employee is at a significant increased risk of percutaneous exposure to an infectious agent transmissible by the bloodborne route when working in a biological safety cabinet. In these cases, strict adherence to mucous membrane protective practices is required. Written by: B. Petrella Page: 10 of 13 Approved by: IBC Approval date: 10/3/18
11 Institutional Biosafety Committee Annual Certification of Biological Safety Cabinets. Annual inspection and certification of biological safety cabinets is the responsibility of the primary investigator. EHS maintains a database of biological safety cabinets and will aid in the scheduling of inspections and certifications. If you need to move your BSC, it must first be professionally decontaminated and recertified once in place. Please contact EHS for assistance (603) Mechanical Pipetting Devices. Mechanical pipetting devices are to be used for all pipetting activities. Mouth pipetting is strictly prohibited. Aerosol-resistant pipette tips are recommended to control carry-over contamination caused by aerosol formation. The tips are ideal for a wide variety of applications including PCR, tissue culture, forensic studies, gel loading, and serological assays as well as pipetting radioactive samples. Sharps Containers. Puncture resistant sharps containers are to be used at all work sites where needles and syringes, Pasteur pipettes, scalpel blades and other sharps are used. Sharps containers should be closed and prepared for disposal when ¾ full. Please refer to the Hazardous Waste Disposal Guide for the correct disposal option for your building. Puncture resistant sharps containers are available through the Scientific Stockrooms. Safer Needle Devices. On April 18, 2001, the Occupational Safety and Health Administration (OSHA) issued the Needlestick Safety and Prevention Act. Congress directed OSHA to make changes to its Bloodborne Pathogens Standard (CFR ) to require the use of safer needle devices when drawing blood. For further information, please contact EHS. You must be approved by EHS in order to conduct blood drawing in your laboratory. Safety Devices for Centrifuges. For low speed centrifugation of infectious materials, safety centrifuge cups must be used. If used, the cups are to be loaded and unloaded only within a biological safety cabinet. High-speed centrifugation of infectious material must be performed using a suitable gasketed rotor that is loaded and unloaded within a biological safety cabinet. Inside a biological safety cabinet, wipe off the exterior of the rotor with a suitable disinfectant before loading and unloading. Vacuum Line Protection. All vacuum lines must be protected by using in-line vacuum HEPA filters and vacuum traps. These disposable filters are available through the stockrooms. In labs where the vacuum lines are used routinely, it is recommended that the filters be changed every six months. In other areas, it is recommended that filters be changed on an annual basis. Immediately change the filter if the system no longer works effectively or becomes overtly contaminated. Vacuum Trap Decontamination. Vacuum traps used for liquid biohazardous waste collection must contain appropriate disinfectants prior to use. Wescodyneâ is Written by: B. Petrella Approved by: IBC Page: 11 of 13 Approval date: 10/3/18
12 recommended since it is a stable disinfectant. The waste must be removed and the flask cleaned whenever ½ volume of flask volume is reached, or every 3 months (which ever comes first). Vacuum trap decontamination schedule stickers must be placed on all vacuum trap flasks to serve as a record of proper disinfection and disposal. These stickers are readily available from EHS as seen here. C. Work Practice Controls Hand Washing. Hand washing is the single most effective means of preventing exposure to, or spread of, infectious agents. Hand washing must be performed after removing gloves, upon completion of a task within a biological safety cabinet, before leaving the lab, when hands are known or suspected to be contaminated and before contact with face or mouth. Hands should be lathered thoroughly with an antimicrobial soap, vigorously washed for approximately seconds then rinsed with copious amounts of water. Use paper towels to turn off the water faucet to avoid immediate recontamination. Food and Consumables. Eating, drinking, smoking, applying cosmetics or lip balm, and handling contact lenses are prohibited in all laboratory settings and other work areas where there is a possibility of occupational exposure. Food and drink shall not be kept in laboratories or refrigerators, freezers, shelves, cabinets or on countertops or bench tops. Sharps Safety. Needles and other sharps must not be bent, recapped, or removed from syringes except when the lab supervisor determines that no alternative is feasible or that such action is required by a specific procedure. Recapping should only be performed using a mechanical recapping device. Shearing or breaking of contaminated needles is prohibited. Aerosols. All procedures involving potentially infectious material shall be performed in such a manner as to minimize splashing, spraying, spattering, and the generation of droplets of these substances. Work shall be conducted in a biosafety cabinet whenever possible to minimize exposures to aerosols. Transport. Transport of biohazardous material outside of the laboratory must be done in secondary containment. If the specimen could puncture the primary container, the primary container shall be placed within a secondary container that is puncture-resistant in addition to the above characteristics. For transport of the material outside of the facility additional requirements apply, contact the EHS office for more information (603) D. Personal Protective Equipment Laboratory Coats. Lab coats are to be worn at all times while working on, or adjacent to, bench top procedures utilizing hazardous chemicals, biological or unsealed radiological materials. Laboratory coats must be appropriately sized for the individual and must be fully buttoned when worn. Sleeves must be of appropriate length as to not expose skin while wearing gloves. Lab coats are not to be taken from the laboratory and brought to non-laboratory areas, especially where food and drink is being prepared or served. It is the responsibility of the Written by: B. Petrella Page: 12 of 13 Approved by: IBC Approval date: 10/3/18
13 PI to ensure all laboratory personnel adhere to Dartmouth s standard of wearing laboratory coats. Gloves. Gloves are to be worn by all employees directly handling biological material or contaminated surfaces. Vinyl examination gloves, surgical synthetic, or N-DEX nitrile gloves may be chosen by the employee based on individual need and preference. Gloves are to be inspected before use and changed routinely. Gloves must be replaced when visibly soiled, torn, or punctured. All gloves must be discarded into an autoclave bag for proper disposal. Information on glove selection and chemical resistance is found on a poster displayed in each laboratory and from EHS. Gloves are never a substitute for thorough hand washing. Hands should be thoroughly washed when entering the lab, after removing gloves and before leaving the lab. Mucous Membrane Protection. Mucous membranes must be protected by wearing a surgical-type mask with safety glasses or a surgical-type mask with attached acetate eye shield when working with infectious or potentially infectious materials outside of a biological safety cabinet. Safety Glasses/Shields. Safety glasses are recommended at all times in the laboratory. Personal Clothes. Any unprotected skin is forbidden. Sandals and other open toed footwear as well as shorts or skirts that leave the skin unprotected are prohibited in all Dartmouth laboratories. Respirators. Respirators must not be used in the laboratory without prior approval from EHS. Contact EHS if you feel respiratory protection is required. Surgical facemasks used for mucous membrane protection are not considered respirators and are not to be used in situations where respiratory protection is required. All respirator users must be enrolled in the Respiratory Protection Program. EHS supplies and maintains the recommended respiratory protective devices. E. Posting Hazard Requirements All labs working with BSL2 agents must post the EHS provided BIOHAZARD sign on the laboratory door. This sign must indicate the specific hazards, PI, and emergency contact information, and should be updated as necessary. The sign is readily available from EHS. Any piece of equipment in common room areas (such as cold room, incubator room, equipment room, etc.) that contains or is used with BSL2 agents must have a biohazard warning label attached. The label must state the biological hazard, date and person of contact. These labels can be obtained from the stockroom or from EHS. Written by: B. Petrella Page: 13 of 13 Approved by: IBC Approval date: 10/3/18
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
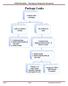 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Medical Waste Management Plan
 Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard
 ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety
 University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety 1.0 Introduction... 1 1.1 Purpose... 1 1.2 Regulatory Background...
University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety 1.0 Introduction... 1 1.1 Purpose... 1 1.2 Regulatory Background...
Hazard Communication Program
 1. Purpose The University of Denver Hazard Communication Program defines the requirements and responsibilities for informing and training employees about workplace hazardous chemicals in accordance with
1. Purpose The University of Denver Hazard Communication Program defines the requirements and responsibilities for informing and training employees about workplace hazardous chemicals in accordance with
What is infection control?
 Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
UPEI Waste Disposal Protocol
 UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
Spring 2005 Pollution Prevention Workshop For Healthcare
 Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Hazard Communication Program
 ILIULIUK FAMILY AND HEALTH SERVICES Hazard Communication Program Reviewed April 2015 TABLE OF CONTENTS SECTION ONE: Staff and General Information Overview 1 I. Introduction II. Purposes III. Responsibility
ILIULIUK FAMILY AND HEALTH SERVICES Hazard Communication Program Reviewed April 2015 TABLE OF CONTENTS SECTION ONE: Staff and General Information Overview 1 I. Introduction II. Purposes III. Responsibility
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN
 ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
Safe Handling and Disposal of Sharps
 SBC Children, Families And Community Health Service Statement of Intent Safe Handling and Disposal of Sharps To provide clear guidelines for the safe handling and disposal of all sharps in order that the
SBC Children, Families And Community Health Service Statement of Intent Safe Handling and Disposal of Sharps To provide clear guidelines for the safe handling and disposal of all sharps in order that the
Section 4 Procedures for Biohazard Control
 Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Welcome to the Hazard Communication Course
 Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
Bloodborne Pathogens Safety in Your Workplace
 Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL. Table of Contents
 EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
GENERAL CHEMISTRY LABORATORY SAFETY
 GENERAL CHEMISTRY LABORATORY SAFETY 1. Read the experiment before coming to Keyes 405. The more prepared you are, the safer and more efficient you will be in lab. 2. Think about what you need to wear to
GENERAL CHEMISTRY LABORATORY SAFETY 1. Read the experiment before coming to Keyes 405. The more prepared you are, the safer and more efficient you will be in lab. 2. Think about what you need to wear to
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
Weber State University Hazard Communication Program April 2000
 Weber State University Hazard Communication Program April 2000 CONTENTS I. Introduction II Listing of Hazardous Materials III. Material Safety Data Sheets IV. Labels and Other Forms of Warning V. Employee
Weber State University Hazard Communication Program April 2000 CONTENTS I. Introduction II Listing of Hazardous Materials III. Material Safety Data Sheets IV. Labels and Other Forms of Warning V. Employee
Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5
 Environmental Health and Safety Manual Policy Number: EH&S 4-4 Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5 PURPOSE: To establish safe handling practices and use of
Environmental Health and Safety Manual Policy Number: EH&S 4-4 Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5 PURPOSE: To establish safe handling practices and use of
Standard Operating Procedure: Hydrofluoric Acid. Copyright Drexel University Health and Safety
 Standard Operating Procedure: Hydrofluoric Acid Copyright Drexel University Health and Safety Hydrofluoric Acid (HF) Purpose of Training The Purpose of this training session is to develop safe working,
Standard Operating Procedure: Hydrofluoric Acid Copyright Drexel University Health and Safety Hydrofluoric Acid (HF) Purpose of Training The Purpose of this training session is to develop safe working,
Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid
 Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid Purpose: This Safe Method of Use applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs),
Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid Purpose: This Safe Method of Use applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs),
MEDICAL WASTE MANAGEMENT PLAN
 MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
