Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
|
|
|
- Kristina Whitehead
- 5 years ago
- Views:
Transcription
1 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including the practices, safety equipment setting, decontamination procedure and etc. There are 4 classification of BSL ranging from a simple teaching laboratory to a most complex safety features to handle the most hazardous microorganism. Each BSL has its own features and recommended safety practices. It is very much different to Biohazard Safety Cabinets (BSC) Class I, II and III. BSC Class I, II and III is the containment level of Biohazard Safety Cabinet alone pertaining to the airflow patterns, HEPA filters and cabinet pressure. BSC is part of the settings of BSL. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other. 36
2 BIOSAFETY LEVEL 1 (BSL-1) BSL-1 is suitable for work involving well-characterized agents not known to cause disease in healthy adults and of minimal potential hazard to lab personnel and the environment and also known as Risk Group 1 organisms. Lab furniture are impervious to water, resistance to all chemicals and medium heat. Enough spaces in between benches and equipment for easy cleaning. No special containment like Biohazard Safety Cabinet is required. Good microbiology practices Lab coat, shoes, gloves and goggle (to prevent splash) Typical setup of a BSL Level 1 laboratory. 37
3 BIOSAFETY LEVEL 2 (BSL-2) Diagnostics and healthcare labs should be designed for BSL-2 to be able to handle all infectious agents under Risk Group 2 that are pathogenic to human but the treatments are available. Lab furniture are impervious to water, resistance to all chemicals and medium heat. Enough spaces in between benches and equipment for easy cleaning. Required to install autoclave for sterilization and decontamination. Containment devices such as Biosafety Cabinet Class I or II need to be installed for all aerosols-producing task. Good microbiology practices Lab coat, shoes, gloves and goggle (to prevent splash) at all times. A typical BLS Level 2 setup - Biohazard sign, Biohazard waste, autoclave, a biosafety cabinet. 38
4 BIOSAFETY LEVEL 3 (BSL-3) The containment laboratory -BSL-3 is designed and provided for work with Risk Group 3 microorganisms and with high volumes or high concentration of Risk Group 2 that pose an increased risk of aerosols spread. Double-entry door, self-closing and interlocking anteroom door allow only one door to open at a time. Surfaces including wall, floor and ceiling panel should be waterresistant and easy to clean. Controlled ventilation system and HEPA-filtered. Autoclave is available in lab for decontamination procedure. Biohazard Safety Cabinet Class II for all manipulation process. Head covers, designated footwears, gloves and respiratory protection must be worn. Medical survellance and immunization program is prepared for the personnel. A typical BLS Level 3 setup - Double entry door, BSC Class II or III 39
5 BIOSAFETY LEVEL 4 (BSL-4) PURPOSE The maximum containment laboratory that is designed for work with Risk Group 4 microorganisms that pose high risk for forming infectious aerosols and life threatening agents. MAIN FEATURE Class III Biohazard Safety Cabinet Double door autoclave for material transfer into/from lab Controlled access Controlled air system SPECIAL PRACTICE Protective suit with self-contain breathing apparatus. Medical survellance and immunization program is prepared for the personnel. Shower decontamination system Double door autoclave for material transfer into/from lab Protective suit with selfa typical BSL 4 shower decontamination contain breathing apparatus 40
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
Section 4 Procedures for Biohazard Control
 Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
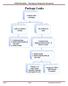 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard
 ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Technical Information. Clorox Healthcare Bleach Germicidal Cleaner OVERVIEW. Clorox Healthcare Bleach Germicidal
 OVERVIEW Bleach Germicidal Cleaner is engineered as a ready-to-use sporicidal cleaner-disinfectant system for healthcare facilities. This EPA-registered disinfectant contains sodium hypochlorite and other
OVERVIEW Bleach Germicidal Cleaner is engineered as a ready-to-use sporicidal cleaner-disinfectant system for healthcare facilities. This EPA-registered disinfectant contains sodium hypochlorite and other
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan
 Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan (last revised Aug 30, 2011) The Maurice Kanbar Center for Biomedical Engineering Room 704 NAB David Wootton, Ph.D. Director
Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan (last revised Aug 30, 2011) The Maurice Kanbar Center for Biomedical Engineering Room 704 NAB David Wootton, Ph.D. Director
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Standard Operating Procedure for disposal of biological waste
 Standard Operating Procedure for disposal of biological waste Effective date: 22 nd March 2018 Review due date: 19 th March 2018 Original Author Name: Rebecca Toone Position: Technician Date: 29.07.2013
Standard Operating Procedure for disposal of biological waste Effective date: 22 nd March 2018 Review due date: 19 th March 2018 Original Author Name: Rebecca Toone Position: Technician Date: 29.07.2013
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
University of Nevada, Reno Operational Plan for Management of Biohazardous Waste
 University of Nevada, Reno Operational Plan for Management of Biohazardous Waste Scope This operational plan covers management of biohazardous waste produced as a result of University of Nevada, Reno (UNR)
University of Nevada, Reno Operational Plan for Management of Biohazardous Waste Scope This operational plan covers management of biohazardous waste produced as a result of University of Nevada, Reno (UNR)
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
MATERIAL SAFETY DATA SHEET TERPINATOR 0-0-4
 MATERIAL SAFETY DATA SHEET TERPINATOR 0-0-4 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: Terpinator 0-0-4 MSDS# Terpinator [1.14] Trade Name: Terpinator Manufacturer s name: Poison Control:
MATERIAL SAFETY DATA SHEET TERPINATOR 0-0-4 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: Terpinator 0-0-4 MSDS# Terpinator [1.14] Trade Name: Terpinator Manufacturer s name: Poison Control:
UNIVERSITY OF CALIFORNIA SANTA BARBARA Medical Waste Management Plan Large Quantity Generator with Onsite Treatment 417
 UNIVERSITY OF CALIFORNIA SANTA BARBARA Large Quantity Generator with Onsite Treatment 417 Contents I. Introduction II. Definitions III. Responsibilities IV. Types and Quantity of Medical Waste Generated
UNIVERSITY OF CALIFORNIA SANTA BARBARA Large Quantity Generator with Onsite Treatment 417 Contents I. Introduction II. Definitions III. Responsibilities IV. Types and Quantity of Medical Waste Generated
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
GENERAL LAB SAFETY RULES. The following is a small list of the rules that MUST be followed in the lab!
 LABORATORY SAFETY GENERAL LAB SAFETY RULES The following is a small list of the rules that MUST be followed in the lab! General Lab Safety Rules: 1. Wear Goggles and Gloves when instructed. - Safety goggles
LABORATORY SAFETY GENERAL LAB SAFETY RULES The following is a small list of the rules that MUST be followed in the lab! General Lab Safety Rules: 1. Wear Goggles and Gloves when instructed. - Safety goggles
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS
 PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
Poison Control: TDD:
 Cutting Edge Solutions, LLC MATERIAL SAFETY DATA SHEET Personal Protective Equipment NFPA HMIS See Section 8 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: Sonoma Gold Bloom MSDS# Sonoma Gold
Cutting Edge Solutions, LLC MATERIAL SAFETY DATA SHEET Personal Protective Equipment NFPA HMIS See Section 8 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: Sonoma Gold Bloom MSDS# Sonoma Gold
Material Safety Data Sheet
 Material Safety Data Sheet NFPA HMIS Personal Protective Equipment COR Health Hazard Fire Hazard See Section 15. Section 1. Chemical Product and Company Identification Common Name/ Trade Name Manufacturer
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment COR Health Hazard Fire Hazard See Section 15. Section 1. Chemical Product and Company Identification Common Name/ Trade Name Manufacturer
EYE- May cause irritation, redness and pain to the eyes. SKIN- May cause irritation and redness at the site of contact.
 Cutting Edge Solutions, LLC MATERIAL SAFETY DATA SHEET Personal Protective Equipment NFPA HMIS See Section 8 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: T-rex MSDS# T-rex [1] Trade Name:
Cutting Edge Solutions, LLC MATERIAL SAFETY DATA SHEET Personal Protective Equipment NFPA HMIS See Section 8 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: T-rex MSDS# T-rex [1] Trade Name:
Poison Control: TDD:
 Cutting Edge Solutions, LLC MATERIAL SAFETY DATA SHEET Personal Protective Equipment NFPA HMIS See Section 8 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: UNCLE JOHN S BLEND MSDS# UNCLE JOHN
Cutting Edge Solutions, LLC MATERIAL SAFETY DATA SHEET Personal Protective Equipment NFPA HMIS See Section 8 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: UNCLE JOHN S BLEND MSDS# UNCLE JOHN
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Poison Control: TDD:
 Cutting Edge Solutions, LLC MATERIAL SAFETY DATA SHEET Personal Protective Equipment NFPA HMIS See Section 8 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: MAG-AMPED MSDS# MAG-AMPED [2] Trade
Cutting Edge Solutions, LLC MATERIAL SAFETY DATA SHEET Personal Protective Equipment NFPA HMIS See Section 8 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: MAG-AMPED MSDS# MAG-AMPED [2] Trade
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET Section 1 - Company and Product Identification Product Name: Level: Part Number: Series Name: Manufacturer: Immunoassay Linearity Set (Thyroid) for use with Siemens Centaur Immunoassay
MATERIAL SAFETY DATA SHEET Section 1 - Company and Product Identification Product Name: Level: Part Number: Series Name: Manufacturer: Immunoassay Linearity Set (Thyroid) for use with Siemens Centaur Immunoassay
KERN HEALTH SYSTEMS POLICIES AND PROCEDURES 2.21-P
 Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
Standard Operating Procedure: Hydrofluoric Acid. Copyright Drexel University Health and Safety
 Standard Operating Procedure: Hydrofluoric Acid Copyright Drexel University Health and Safety Hydrofluoric Acid (HF) Purpose of Training The Purpose of this training session is to develop safe working,
Standard Operating Procedure: Hydrofluoric Acid Copyright Drexel University Health and Safety Hydrofluoric Acid (HF) Purpose of Training The Purpose of this training session is to develop safe working,
Material Safety Data Sheet
 Material Safety Data Sheet NFPA HMIS Personal Protective Equipment 2 Health Hazard Fire Hazard 2 See Section 15. Section 1. Chemical Product and Company Identification Common Name/ Trade Name Manufacturer
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment 2 Health Hazard Fire Hazard 2 See Section 15. Section 1. Chemical Product and Company Identification Common Name/ Trade Name Manufacturer
ATS-SOI-5731 Page: 1 of 5. Approval Block. Prepared by: Signature Date Margaret Crouse 18 JUN Reviewed by: Signature Date
 ATS-SOI-5731 Page: 1 of 5 Approval Block Prepared by: Signature Date Margaret Crouse 18 JUN 2014 Reviewed by: Signature Date Brian Flynn 18 JUN 2014 Approved by: Signature Date Kristal Jewell 18 JUN 2014
ATS-SOI-5731 Page: 1 of 5 Approval Block Prepared by: Signature Date Margaret Crouse 18 JUN 2014 Reviewed by: Signature Date Brian Flynn 18 JUN 2014 Approved by: Signature Date Kristal Jewell 18 JUN 2014
This policy applies to all Pharmacy or non-pharmacy personnel accessing controlled work areas located within Lower Mainland Pharmacy Services.
 Page 1 of 13 BACKGROUND Proper hand hygiene and garbing are an essential part of the sterile compounding process to prevent patient harm from contaminated compounded sterile preparations and protect staff
Page 1 of 13 BACKGROUND Proper hand hygiene and garbing are an essential part of the sterile compounding process to prevent patient harm from contaminated compounded sterile preparations and protect staff
Instructor Guide. Georgia Department of Technical and Adult Education. Decontamination and Infection Control
 Instructor Guide Georgia Department of Technical and Adult Education Decontamination and Infection Control Copyright October 2002 by. All rights reserved. No part of this manual may be reproduced or transmitted
Instructor Guide Georgia Department of Technical and Adult Education Decontamination and Infection Control Copyright October 2002 by. All rights reserved. No part of this manual may be reproduced or transmitted
SAFETY DATA SHEETS. This SDS packet was issued with item:
 SAFETY DATA SHEETS This SDS packet was issued with item: 078080905 The safety data sheets (SDS) in this packet apply to the individual products listed below. Please refer to invoice for specific item number(s).
SAFETY DATA SHEETS This SDS packet was issued with item: 078080905 The safety data sheets (SDS) in this packet apply to the individual products listed below. Please refer to invoice for specific item number(s).
DIVISION OF MATHEMATICS, SCIENCE & ENGINEERING
 DIVISION OF MATHEMATICS, SCIENCE & ENGINEERING Formaldehyde Safety Program Updated March, 2014 A. PURPOSE 1. The purpose of this program is to protect employees from the hazards associated with formaldehyde
DIVISION OF MATHEMATICS, SCIENCE & ENGINEERING Formaldehyde Safety Program Updated March, 2014 A. PURPOSE 1. The purpose of this program is to protect employees from the hazards associated with formaldehyde
PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS
 PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS CESafety 1 WHMIS CESafety 2 What is WHMIS? WHMIS is a Canada-wide system designed to give employers and workers information about hazardous materials
PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS CESafety 1 WHMIS CESafety 2 What is WHMIS? WHMIS is a Canada-wide system designed to give employers and workers information about hazardous materials
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES
 BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
Cephalexin Capsules 250mg & 500 mg
 Orchid Healthcare Material Safety Data Sheet Distributed by: Karalex Pharma, LLC 470 Chestnut Ridge Road Woodcliff Lake, NJ 07677 Phone: 201-529-0402 Cephalexin Capsules 250mg & 500 mg SECTION 1 - CHEMICAL
Orchid Healthcare Material Safety Data Sheet Distributed by: Karalex Pharma, LLC 470 Chestnut Ridge Road Woodcliff Lake, NJ 07677 Phone: 201-529-0402 Cephalexin Capsules 250mg & 500 mg SECTION 1 - CHEMICAL
Material Safety Data Sheet
 Material Safety Data Sheet NFPA HMIS Personal Protective Equipment Health Hazard Fire Hazard 2 See Section 5. Section. Chemical Product and Company Identification Common Name/ Trade Name Manufacturer Commercial
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment Health Hazard Fire Hazard 2 See Section 5. Section. Chemical Product and Company Identification Common Name/ Trade Name Manufacturer Commercial
Material Safety Data Sheet
 Material Safety Data Sheet NFPA HMIS Personal Protective Equipment 2 Health Hazard Fire Hazard 2 See Section 15. Section 1. Chemical Product and Company Identification Common Name/ Trade Name Manufacturer
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment 2 Health Hazard Fire Hazard 2 See Section 15. Section 1. Chemical Product and Company Identification Common Name/ Trade Name Manufacturer
Material Safety Data Sheet
 Material Safety Data Sheet NFPA HMIS Personal Protective Equipment 0 Health Hazard Fire Hazard 0 See Section 5. Section. Chemical Product and Company Identification Common Name/ Trade Name a-amylase Catalog
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment 0 Health Hazard Fire Hazard 0 See Section 5. Section. Chemical Product and Company Identification Common Name/ Trade Name a-amylase Catalog
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
