Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
|
|
|
- Anis Joseph
- 6 years ago
- Views:
Transcription
1 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of Canada (PHC) and Queen s University policies. This checklist is to be filled out by the Principal nvestigator, or designate, as a self-audit prior to a biohazard lab inspection. nswer each question prior to the inspection by ticking, Yes or No, and present the completed form to the inspectors when they arrive. Fill this form out for your main lab and begin the inspection there. short version of the form is available for your other labs. The person who filled out the form is to be present at the inspection to discuss answers on the form with the inspectors. Other lab members are welcome to attend the inspection. Training records are to be available in one of your laboratories for review by the inspectors. They may be stored elsewhere at other times. nvestigator: Secondary Biohazard Contact: Person completing self-audit: Building & Room Biohazard Containment Level Biohazard Committee nspection Team: Signatures: nspection Date: Comments for the attention of the lab and/or the University Biosafety Officer: bbreviations:, not applicable, Yes, compliance; No, compliance lacking; V, nspectors Verified at nspection;, required only in lab; CBS requirement number from Canadian Biosafety Standard, 2 nd Edition. Where the containment level is not indicated, the requirement applies to all biohazard labs. 1. Biohazardous Material nformation 1.1 What are the biohazardous materials used in this lab? General types of material (as listed on biohazard sign) : 1.2 s an inventory of biological agents handled or stored in the containment zone, maintained and kept up to date? Note: the inventory must contain a list of all agents and materials, their risk group, source and the rooms or locations in which they are used or stored. Quantities are not required. nventory must be kept up to date in biohazard permit in TRQ/Romeo.
2 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members 1.3 Transfer of biohazardous material to another research group / individual / company, either at Queen s or outside the University, is reported to the Biosafety Officer prior to such transfer to ensure that all the appropriate safety and regulatory requirements are met. 2. Signage 2.1 Biosafety warning sign posted on laboratory door indicates containment level. 2.2 Sign has current contact information for the supervisor and other responsible person (usually the secondary biohazard contact). 2.3 Sign lists types of biohazardous material (eg. RG1 bacteria (cloning strains only), RG1 bacteria (opportunistic infection risk), RG1&2 mammalian cell lines, RG2 amphotropic retrovirus). 2.4 re there any special provisions for entry beyond general level 2 provisions? (e.g. immunizations, health restrictions); relevant information is included on the biohazard sign on the door. Comment re signage: 3. General Lab Facilities and Procedures 3.1 ccess to the laboratory is at the discretion of the laboratory director (children should not be present in laboratory areas). 3.2 Trainees and visitors must be accompanied by a trained staff member. 3.3 Door to the laboratory kept closed. 3.4 Lab kept clean and tidy. No cardboard boxes on the floor. 3.5 Visual inspections of the containment zone to be conducted in order to identify faults and/or deterioration; when found, corrective actions to be taken. Lab benches, floor, equipment, etc. are in good condition, with surfaces and caulking intact, so that they can be readily decontaminated. 3.6 ll spills, accidents and overt or potential exposures must be reported promptly in writing to the Departmental Safety who is: 3.7 Emergency Plan posted in the laboratory is current (updated and reposted annually at the time of annual retraining) familiar to all personnel includes site specific information on spill clean-up, fire, and where applicable, BSC failure, animal escape, etc. 3.8 Eyewash in accordance with containment zone activities (or, depending on the hazard, eyewash in hall within 10 seconds access and no more than one door); access not obstructed; tested weekly and card initialled. 3.9 Safety shower in accordance with containment zone activities within 10 seconds access time and through no more than one door Sink identified for hand washing has soap and paper towels; if lab has more than one sink and if feasible then dedicate sink near lab exit for hand washing only; if hand washing sink is not near the exit then a sign must be posted near the exit to remind personnel to wash their hands.
3 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members 3.11 Personnel are to wash hands after completing tasks that involve the handling of infectious material or toxins and before undertaking other tasks in the containment zone. Hands washed after removal of gloves, after handling potentially biohazardous material, immediately before leaving the laboratory Eating, drinking, smoking, storing food or utensils, applying cosmetics and inserting or removing contact lenses is not permitted Paperwork and computers kept separate from biohazardous materials work areas. f the desk is on a bench beside where work with biohazards is done, without a change in the height to separate the desk area, then there is a line of tape on the bench to indicate the clean area Long hair tied back so as not to contact hands, specimens, containers or equipment ll pipetting using automatic pipets (No oral pipetting) Creation of aerosols and their effects minimized indicate method for different procedures in use (e.g. during pipetting, vortexing, centrifuging, for sonicating) Traffic flow patterns from areas of lower contamination (i.e., clean) to areas of higher contamination (i.e., dirty) to be established and followed, as determined by a local risk assessment (LR). CBS requirement to limit the spread of contamination Two-way communication system(s) to be provided inside the containment barrier that allows communication between inside the containment barrier to outside the containment zone, in accordance with function. (e.g. a phone, or a window in door to permit communication through a window (e.g., using notes and signs, or hand signals). CBS requirement to facilitate response in an emergency and to reduce traffic in and out of containment zone Centrifugation performed in closed containers (tubes) to contain aerosols. Tubes are opened only in the biological safety cabinet unless risk assessment indicates otherwise (and approved written operational procedures are in place) Lids for centrifuge buckets that are aerosol resistant are used for level 2 material that is known to be infectious (e.g. blood from individuals known to be infected with a blood borne pathogen, risk group 2 infectious pathogenic bacteria and viruses). O-rings are checked routinely and replaced when they are cracked or appear dried out Vacuum aspiration equipment is protected with a HEP filter as per SOP-Biosafety-01 (available in Botterell biobar) Leak-proof containers are used for transport of infectious materials between labs. i.e. double contained. Procedures, as determined by a LR, to be in place to prevent a leak, drop, spill, or similar event during the movement of infectious material or toxins within the containment zone or between containment zones within a building Biohazard bags are supported in solid containers that have a biohazard symbol Use of needles, syringes, and other sharp objects is strictly limited and avoided when suitable alternatives are available Bending, shearing, re-capping, or removing needles from syringes is avoided, and, when necessary, performed only as specified in written SOPs. Comment re lab facilities and procedures: 4. Biological Safety Cabinet (BSC) 4.1 ware of SOP-Biosafety-03 Biological Safety Cabinets.
4 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members 4.2 ntake and rear grilles are clear of obstructions. BSC is not overcrowded and only equipment and supplies needed immediately for the work being done are in the BSC. 4.3 Work surfaces and under front grill are clean and free of visible biological residue. 4.4 Bunsen burners and/or open flames are not used in biological safety cabinets. Open flames are not permitted inside BSCs; consider an alternative, such as an electrical bacticinerator. 4.5 Biosafety Cabinet located away from high traffic areas, doors, and air supply/exhaust diffusers? 4.6 Procedures to be followed to prevent the inadvertent spread of contamination from items removed from the BSC after handling infectious material or toxins. (i.e. everything surface decontaminated before being removed from the BSC) 4.7 BSC used for procedures that may produce infectious aerosols and that involve high concentrations or large volumes, (unless a risk assessment in consultation with the University Biological Safety Officer/Biohazard Committee has indicated otherwise). 4.8 BSCs to be certified upon initial installation, annually, and after any repairs, modification, or relocation. Date for next annual BSC certification: Comment re BSC: 5. Personal Protective Equipment 5.1 Fastened lab coat worn. 5.2 Dedicated lab coat for level 2 work. 5.3 Lab coat stored separately from street clothing and not on top of each other on hooks. 5.4 Lab coat removed prior to entering non-laboratory areas. 5.5 How and where are lab coats laundered? 5.6 f a known or suspected exposure occurs is contaminated clothing decontaminated before laundry? How? 5.7 Closed toe and heal footwear worn by all personnel. Type of footwear worn to be selected to prevent injuries and incidents, in accordance with containment zone function. 5.8 Suitable eye and face protection when required (check availability of goggles &/or face shield). 5.9 Contact lenses worn only when other corrective eyewear is not suitable and if worn then other eye protection is worn when there is a splash risk Gloves worn for work with infectious agents, toxins, blood and other potentially biohazardous material Open wounds, cuts, and breaks in the skin should be covered with a waterproof dressing Glove material not permeated by substances used in conjunction with biohazards (e.g. chemical hazards, cancer chemotherapeutics) Gloves to be removed prior to leaving laboratory (or one glove method if carrying hazardous materials) f N95 respirators are required, all users have been fit tested through EH&S every 2 years.
5 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members 5.15 written donning and doffing procedure for the particular PPE worn in your laboratory must be developed and posted. See Queen s Biosafety Manual 2013 page 44 and 25 for an example. Comment re PPE: 6. Storage, Decontamination and Disposal 6.1 Containers of pathogens, toxins, or other regulated infectious material stored outside the containment zone to be: n containers that are labelled, leakproof, and impact resistant kept either in locked storage equipment or within an area with limited access (e.g. a corridor on a floor where the door is always locked). Storage locations outside of the containment zone are noted on the inventory (and thereby on the biohazard permit). 6.2 Gross contamination to be removed prior to decontamination of surfaces and equipment, and disposed of accordingly. i.e. clean to remove most of the organic matter so that chemical decontamination is effective. 6.3 Decontamination to be performed with a disinfectant effective against the pathogen(s) in use, or a neutralizing chemical effective against the toxin(s) in use, at a frequency to minimize the potential of exposure to infectious material or toxins. 6.4 Equipment, supplies, wastes, etc. are disinfected prior to removal from the laboratory or if waste is being removed for decontamination or disposal through EH&S, then double contained and surface decontaminated. 6.5 ll biohazardous material decontaminated prior to disposal (or disposed as hazardous waste through EH&S). Contaminated aqueous liquids to be decontaminated prior to release to sanitary sewers. indicate method(s): 6.6 f autoclaves are used for decontamination, lab is aware of SOP-Biosafety-09 utoclaves Biohazardous Waste Treatment and biological indicators (Bacillus stearothermophilus spores) are used weekly to monitor efficacy in a representative waste load. 6.7 Name of person responsible for biological indicator testing: 6.8 Biohazard labels, if present, are defaced after autoclave decontamination and prior to disposal. (do not use red biohazard bags with a printed biohazard label for waste that will be autoclaved and discarded in the municipal waste) 6.9 Biohazardous material contaminated with chemical hazards or radioisotopes is disposed through EH&S. Human and animal tissues are disposed through EH&S for incineration Bench coat (paper backed with plastic) may be used to contain hazardous material. f used it is changed regularly & not taped to benches Contaminated sharps are placed in an approved labelled puncture-proof disposable container for decontamination. Comment re storage, decontamination & disposal:
6 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members 7. Training 7.1 Lab biosafety information is available including Queen s Biosafety Policies and Manual 2013 (check there should be either a link on computer or a hardcopy; confirm that lab members know how to find their biohazard permit and SOPs either in the lab or in TRQ/Romeo). 7.2 ll staff/students have received the appropriate training; check that training record was signed by the trainee and the supervisor (or designate). Training records should be organized with those for each individual together. Training records for those still working in the lab should be in a group. Records for those who have left the lab should be grouped separately and retained for 5 years. 7.3 Lab is aware that everyone listed on a biohazard permit is to complete the appropriate Queen s EH&S Biosafety Training quiz (level 1 or level 2). 7.4 Refresher training on emergency response procedures is provided annually and documented in the lab. Comment re training: 8. Medical Surveillance 8.1 Lab is aware that in an Emergency they should go to KGH Emergency; that Walsh and ssociates is the Occupational Health Services provider for Queen s (no longer KGH OHS); Walsh and ssociates will provide appropriate follow-up care after an incident Walsh and ssociates will provide any medical surveillance for the lab as specified in the biohazard permit e.g. immunizations, titre checks, etc. 8.2 mmunizations required for work in the lab? 8.3 ny specific immune-surveillance or incident response info required and posted? E.g. if human blood, tissues or bodily fluids are used, the lab is aware of SOP-Biosafety-08; has posted the first aid response to an exposure incident and a map to KGH Emergency and contact information for Walsh and ssociates OHS Comment re medical surveillance:
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
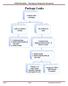 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Medical Waste Management Plan
 Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
APPROVAL REVIEW PROCEDURES
 Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard
 ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
Permanent Body Art Facility Plan Review Application
 Permanent Body Art Facility Plan Review Application Livingston County Health Department 2300 East Grand River Suite 102, Howell, MI 48843 Ph:517-546-9858 Fx:517-546-9853 www.lchd.org Authority - Michigan
Permanent Body Art Facility Plan Review Application Livingston County Health Department 2300 East Grand River Suite 102, Howell, MI 48843 Ph:517-546-9858 Fx:517-546-9853 www.lchd.org Authority - Michigan
BODY ART ESTABLISHMENT PLANNING APPLICATION
 BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Queen's University Technicians Position Description Questionnaire. Immediate Supervisor: Manager, Biohazard, Radiation and Chemical Safety
 Queen's University Technicians Position Description Questionnaire Field of Work: Safety Technician (Biohazard and Chemical Safety) Name: TBA Department: Environmental Health & Safety Date: June 22, 2018
Queen's University Technicians Position Description Questionnaire Field of Work: Safety Technician (Biohazard and Chemical Safety) Name: TBA Department: Environmental Health & Safety Date: June 22, 2018
Safe Handling and Disposal of Sharps. Reference Guide
 Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Hazard Communication Program
 1. Purpose The University of Denver Hazard Communication Program defines the requirements and responsibilities for informing and training employees about workplace hazardous chemicals in accordance with
1. Purpose The University of Denver Hazard Communication Program defines the requirements and responsibilities for informing and training employees about workplace hazardous chemicals in accordance with
University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety
 University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety 1.0 Introduction... 1 1.1 Purpose... 1 1.2 Regulatory Background...
University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety 1.0 Introduction... 1 1.1 Purpose... 1 1.2 Regulatory Background...
Welcome to the Hazard Communication Course
 Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Section 4 Procedures for Biohazard Control
 Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
UPEI Waste Disposal Protocol
 UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
AFS Environmental Health & Safety Conference Nashville, TN August 24, 2010
 AFS Environmental Health & Safety Conference Nashville, TN August 24, 2010 Protect employees from illness and injury associated with the use of hazardous substances A generic and performance oriented standard
AFS Environmental Health & Safety Conference Nashville, TN August 24, 2010 Protect employees from illness and injury associated with the use of hazardous substances A generic and performance oriented standard
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS
 PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS CESafety 1 WHMIS CESafety 2 What is WHMIS? WHMIS is a Canada-wide system designed to give employers and workers information about hazardous materials
PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS CESafety 1 WHMIS CESafety 2 What is WHMIS? WHMIS is a Canada-wide system designed to give employers and workers information about hazardous materials
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BODY ART FACILITY CONSTRUCTION PLAN CHECK
 BODY ART FACILITY CONSTRUCTION PLAN CHECK Type of Facility: (mark one) Permanent Temporary/Special Event Are you a: (mark one) New Facility Existing with new ownership Existing Facility Existing remodel
BODY ART FACILITY CONSTRUCTION PLAN CHECK Type of Facility: (mark one) Permanent Temporary/Special Event Are you a: (mark one) New Facility Existing with new ownership Existing Facility Existing remodel
MEDICAL WASTE MANAGEMENT PLAN
 MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
DIVISION OF MATHEMATICS, SCIENCE & ENGINEERING
 DIVISION OF MATHEMATICS, SCIENCE & ENGINEERING Formaldehyde Safety Program Updated March, 2014 A. PURPOSE 1. The purpose of this program is to protect employees from the hazards associated with formaldehyde
DIVISION OF MATHEMATICS, SCIENCE & ENGINEERING Formaldehyde Safety Program Updated March, 2014 A. PURPOSE 1. The purpose of this program is to protect employees from the hazards associated with formaldehyde
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES
 BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
Prepared by Laurel Arrigona, Matt Bavougian, Michael Crea, John Johnson, Steve Joyner, Sarah Robbin, and KC Stevenson
 122 nd AFDO Educational Conference Burlington, Vermont Body Art Committee June 10, 2018 Prepared by Laurel Arrigona, Matt Bavougian, Michael Crea, John Johnson, Steve Joyner, Sarah Robbin, and KC Stevenson
122 nd AFDO Educational Conference Burlington, Vermont Body Art Committee June 10, 2018 Prepared by Laurel Arrigona, Matt Bavougian, Michael Crea, John Johnson, Steve Joyner, Sarah Robbin, and KC Stevenson
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL. Table of Contents
 EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5
 Environmental Health and Safety Manual Policy Number: EH&S 4-4 Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5 PURPOSE: To establish safe handling practices and use of
Environmental Health and Safety Manual Policy Number: EH&S 4-4 Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5 PURPOSE: To establish safe handling practices and use of
ATS-SOI-5731 Page: 1 of 5. Approval Block. Prepared by: Signature Date Margaret Crouse 18 JUN Reviewed by: Signature Date
 ATS-SOI-5731 Page: 1 of 5 Approval Block Prepared by: Signature Date Margaret Crouse 18 JUN 2014 Reviewed by: Signature Date Brian Flynn 18 JUN 2014 Approved by: Signature Date Kristal Jewell 18 JUN 2014
ATS-SOI-5731 Page: 1 of 5 Approval Block Prepared by: Signature Date Margaret Crouse 18 JUN 2014 Reviewed by: Signature Date Brian Flynn 18 JUN 2014 Approved by: Signature Date Kristal Jewell 18 JUN 2014
