ECU Radiation, Biosafety and Hazardous Substances Committee
|
|
|
- Archibald Davis
- 6 years ago
- Views:
Transcription
1 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure must be followed Definitions Aliquot a portion of larger whole Aliquoting to divide a larger whole into portions ECU Edith Cowan University First morning urine sample a urine specimen collected when the participant first wakes in the morning NHMRC National Health and Medical Research Council Mid-stream urine sample - a urine specimen collected during the middle of a flow of urine, after the urinary opening has been carefully cleaned OSH Occupational Safety and Health PPE Personal Protective Equipment Random urine sample a urine specimen collected at anytime RBHSC Radiation, Biosafety and Hazardous World Health Organisation 24 hour urine sample urine collected for an exact period of 24 hours Legislative and ECU AS Safety in laboratories - Microbiological safety and containment requirements ECU Immunisation Guideline ECU Human Biomedical Products Collected from External Agencies and Institutions ECU Handling Infectious Materials and Infection Control ECU Human Ethics Page 1 of 7
2 Task Location/s NHMRC Australian Guidelines for the Prevention and Control of Infection in Healthcare 2010 A1.2.1 Standard precautions and Part B: Standard and Transmission- Based Precautions WHO Infection controls standard precautions in health care Locations approved by the RBHSC and that satisfy physical containment level 2 (PC2) requirements. Contact details for: Before commencing this procedure establish the Laboratory Manager of the Area Before commencing this procedure establish the Supervisor of this Activity Risk Assessment and Study Specific Protocol Equipment used Training A task risk assessment has been performed in order to create this standard operating procedure, additional specific study information should be documented and used in conjunction with this SOP. If you are not conducting the task line with this procedure please complete a new or updated task risk assessment and take it to your supervisor for review and approval in line with the risk acceptance criteria. Class II Biological safety cabinet Sample containers Sample tubes Pipettor and rack Sterile pipette tips Sterile disposable pipettes Freezer storage box with lid Biohazard bags zip-lock Biohazard bags large with twist ties Biogram and waste urine container Bench coat Fine tip cryo pen Biohazard sharps disposal bin Biohazard waste disposal bin lined with a biohazard bag( large and small) Vinyl laboratory stool 4 C laboratory fridge - 80 o C Freezer 70% ethanol spray bottle Disposal trolley Urine disposal receptacle i.e. Toilet, pan flusher or flushing sluice Spill Kit scoop/scraper, disposable gloves, protective apron, surgical mask, safety glasses, absorbent agent, biohazard waste bags and ties and detergent( all parts should be disposable to avoid cross contamination) Study specific equipment outlined in study specific protocol Aseptic Techniques Biological Safety Training Hazardous Substances Training Laboratory Induction and Access Page 2 of 7
3 Procedure Steps ECU Radiation, Biosafety and Hazardous OSH Induction PC2 Training Specific Laboratory Equipment Training according to SOPs for the specific equipment( i.e. Class II Biological Safety Cabinet, Autoclave, Cryogenics, Pipetting) Standard(Universal) Precautions Study Specific Protocols Step 1. Health and Safety Requirements 1.1 Ensure you have the recommended immunisations in accordance with ECU Immunisation Guideline. 1.2 Report all hazards and incidents to your Supervisor and the Laboratory Manager (including equipment faults) and in accordance with the ECU Incident Reporting and Investigation Guideline. 1.3 Use aseptic techniques at all times. 1.4 Manage biological spills and other microbiological laboratory disinfection according to requirements refer to TABLE F1 AS Safety in laboratories Microbiological safety and containment. 1.5 Ensure chemicals being used are approved for use in the laboratory and for the task you are completing, read the chemical risk assessment and safety data sheet. 1.6 Keep the work area clean and tidy at all times. 1.7 Dispose of any biological sharps in biological sharps bin. 1.8 Do not leave caps/lids on the bench. The caps/lids should remain under your control throughout the transfer. Procedure/s 1.9 No eating, drinking, chewing or application of lipstick or lip salve in the laboratory. Step 2. Technical Preparation and Laboratory Workspace Setup 2.1 Long hair must be tied up to prevent contamination of and from the samples you are working with. 2.2 Process participant samples on the day they are received or as outlined in the study specific protocol. 2.3 Ensure that you are wearing closed-in footwear, disposable laboratory coat and safety glasses. 2.4 Wash your hands and put on your disposable nitrile or latex gloves. 2.5 Determine what sample tubes are required for each test and the operating Page 3 of 7
4 temperature required for aliquoting i.e. room temperature for 30 minutes or 4 o C. 2.6 Label all participant collection sample containers according to study specific protocol. 2.7 Label all participant study sample tubes according to study specific protocol, ensure samples are de-identified but clearly state the type of sample they contain i.e. human serum. 2.8 Prepare the biological safety cabinet according to its SOP, including cleaning the bench with 70% ethanol solution in spray bottle before working on it (never leave 70% ethanol solution spray bottle on the same bench as a flammable source). NOTE: All work involving unsealed biological samples must only take place in the biological safety cabinet. 2.9 It the biological safety cabinet SOP requires, lay a bench coat in the biological safety cabinet to cover the workspace. Ensure that the absorbent side (non-shiny) is facing up Place the biohazard sharps disposal bin and any other study specific equipment needed to aliquot the unsealed biological samples inside the biological safety cabinet i.e. labelled freezer storage container, sample tubes, pipette, pipette tips and rack, Biogram waste container. Step 3. Sample Aliquoting 3.1 Dependent on the type of biological safety cabinet being used, the height of the person working and the length of the procedure, either sit on a vinyl laboratory stool or stand, while work is taking place in the cabinet. 3.2 Each participant s collection sample container should be processed separately. Individual processing of each participant s collection sample container is important as it prevents accidental swapping of samples. 3.3 Inside the biological cabinet, for each type of study sample aliquot, prepare the pipettor measurement according to the study specific protocol and attach a sterile pipette tip or use a sterile disposal pipette. First morning urine sample, Mid-stream urine sample, Random urine sample 3.4 Inside the biological cabinet gently invert the urine specimen collection container, place it on the bench coat (if used). Gently open each participant s sample container. Using the pipette, draw the appropriate amount of sample from the sample container and transfer to the appropriate study sample tube. Recap as you go. Use the same sterile pipette for all draws from the sample container. Page 4 of 7
5 3.5 Place the sample tube into the freezer storage box. 3.6 Place the used pipette/pipette tip into the small biohazard waste disposal bin lined with a biohazard bag in the biological cabinet. 3.7 While working in the biological cabinet, pour the remaining urine sample into the designated Biogram waste container. Place the recapped sample containers back into the biohazard zip lock bag and into the small biological waste bin. 3.8 Repeat steps 3.4 to 3.8 for subsequent participant urine samples, one participant at a time. 3.9 Place the freezer storage container (containing the aliquotted sample tubes) into the appropriate freezer in their specified tray numbers as per the study specific protocol Place the bench coat in the biohazard waste disposal bin lined with a biohazard bag (small) Securely tie the biohazard bag in the small biohazard waste disposal bin inside the biological cabinet. Place the tied bag into another clean biohazard bag and securely tie it and place it in the large biohazard bin. Contact the Laboratory Manager to establish the days and times that the large biohazard bin will be emptied Securely recap the Biogram waste collection container and place on a trolley, transport to the study specific urine disposal receptacle i.e. toilet, pan flusher or flushing sluice. Note: Remember to remove gloves when opening doors, using the laboratory telephone or pressing lift buttons to gain access to areas outside the laboratory Return the emptied Biogram waste collection container to the laboratory Replace all biohazard bin liners once emptied. 24 hour urine 3.15 Place the trolley with the 24 hour urine samples on it next to the biological safety cabinet Inside the biological cabinet each participant s 24 hour urine collection bottle is weighed on the allocated scales. The results including any comments (i.e. more than one sample bottle used) are recorded and any calculations made as per the study specific protocol. Page 5 of 7
6 3.17 Inside the biological cabinet, ensure the cap of the 24 hour urine collection bottle is securely screwed on and mix the urine sample by gently inverting the bottle 5 times, holding the bottle securely with one hand and the cap with the other If the urine is contained in more than one bottle, another laboratory assistant will be required to assist with securing bottles while the urine is mixed between the sample bottles as per the study specific protocol. Mixing of samples must occur in the biological cabinet In the biological cabinet, get the chosen pipette ready, unscrew the cap for the sterile screw top sample container and place it on the bench. Ensure the cap is facing up at all times Unscrew the cap of the prepared 24 hour urine collection bottle as per the study specific protocol. Proceed to aliquot the study specific amount of urine into the labelled sterile screw top container Screw the cap back onto the 24 hour urine collection bottle for that participant and place it back into the storage container it arrived in and onto the trolley ready for disposal Using the same pipette for the sample, transfer the study specific amounts of urine into the required sample containers. Recap as you go. Place aliquotted samples into study specific storage containers and store as outlined in study specific protocol Dispose of the used pipette/pipette tips in small biological waste bin in the biological cabinet While working in the biological cabinet, pour the remaining urine sample from labelled sterile screw top container into the designated Biogram waste container Once all 24 hour urine samples have been aliquotted, the recapped 24 hour urine collection bottles should be placed in the cooler bags on the trolley ready to be disposed of in the study specific urine disposal receptacle i.e. toilet, pan flusher or flushing sluice. Note: Spills of potentially infectious materials should be promptly cleaned as follows: Access laboratory spill kit Put all PPE contained in the kit on Confine and contain the spill clean visible matter with disposable absorbent material and place all materials in the biohazard waste bin Clean the spill area with a cloth or paper towel using detergent solution ( use chemical disinfectants such as sodium hypochlorite based on assessment of risk of transmission of infectious agents from that spill Page 6 of 7
7 Step 4. Laboratory Take Down 4.1 Clean bench with 70% ethanol solution in spray bottle after working on it (never leave 70% ethanol solution spray bottle on the same bench as a flammable source). 4.2 Shut down the Biological Cabinet according to the SOP for shutting down. 4.3 After completing your work with the samples, remove your laboratory coat and gloves carefully and wash your hands thoroughly. CONTACT INFORMATION Procedure Owner All Enquiries Contact: address: Chair Radiation Biosafety Hazardous Radiation Biosafety Hazardous RBHSC@ads.ecu.edu.au APPROVAL HISTORY Procedure Approved by: Chair Radiation Biosafety Hazardous Date Procedure First Approved: January 2017 Date last modified: January 2017 Revision History: V01.03 Procedure first approved document Next Revision Due: January 2020 HPRM File Reference HSMS/84 Page 7 of 7
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
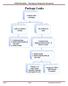 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
LYMPHOCYTE ISOLATION
 Blood tubes to be processed for Lymphocyte Isolation and/or Lymphocyte Transformation should be placed in the rack located on top of the black cabinet and stored at room temperature. A. WARM WASH MEDIUM
Blood tubes to be processed for Lymphocyte Isolation and/or Lymphocyte Transformation should be placed in the rack located on top of the black cabinet and stored at room temperature. A. WARM WASH MEDIUM
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Infection Prevention Guidelines. Safe Use, Handling & Disposal of Sharps
 Infection Prevention Guidelines Safe Use, Handling & Disposal of Sharps Author: Anne Tateson Owner: Infection Prevention Team Publisher: Infection Prevention Date of Version Issue: February 2015 Version:
Infection Prevention Guidelines Safe Use, Handling & Disposal of Sharps Author: Anne Tateson Owner: Infection Prevention Team Publisher: Infection Prevention Date of Version Issue: February 2015 Version:
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
BODY ART FACILITY PLAN REVIEW OVERVIEW
 BODY ART FACILITY PLAN REVIEW OVERVIEW The City of Pasadena Public Health Department, Environmental Health Division shall issue a health permit for a body art facility after an investigation has determined
BODY ART FACILITY PLAN REVIEW OVERVIEW The City of Pasadena Public Health Department, Environmental Health Division shall issue a health permit for a body art facility after an investigation has determined
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Aseptic Milk Sampling for Bacteriology
 Aseptic Milk Sampling for Bacteriology Year Group: BVSc1 + Document number: CSL_F02 Equipment for this station: Equipment list: Cow model (fitted with milking udder) Wet wipes Paper towel Teat dip bottle
Aseptic Milk Sampling for Bacteriology Year Group: BVSc1 + Document number: CSL_F02 Equipment for this station: Equipment list: Cow model (fitted with milking udder) Wet wipes Paper towel Teat dip bottle
Acid Or Alkali? Testing With Cabbage
 Acid Or Alkali? Testing With Cabbage Topic Using vegetables as an acid/base indicator Introduction Forensic scientists need to discover if someone has tampered with liquids (e.g., cosmetics, cleaning products,
Acid Or Alkali? Testing With Cabbage Topic Using vegetables as an acid/base indicator Introduction Forensic scientists need to discover if someone has tampered with liquids (e.g., cosmetics, cleaning products,
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
2.6-1 SCIENCE EXPERIMENTS ON FILE Revised Edition. Cloud Chamber
 2.6-1 SCIENCE EXPERIMENTS ON FILE Revised Edition Cloud Chamber Topic Cloud formation Time 1 hour! Safety Please click on the safety icon to view safety precautions. Do not touch dry ice with bare hands.
2.6-1 SCIENCE EXPERIMENTS ON FILE Revised Edition Cloud Chamber Topic Cloud formation Time 1 hour! Safety Please click on the safety icon to view safety precautions. Do not touch dry ice with bare hands.
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015
 PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015 Personnel Maija Bluma (573-651-2069; mbluma@semo.edu) is the Microbiology Preparation
PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015 Personnel Maija Bluma (573-651-2069; mbluma@semo.edu) is the Microbiology Preparation
Urine Collection Kit. just between us. This kit contains: Before you begin! Access Key label here
 just between us Urine Collection Kit Before attempting to collect your sample, please read these instructions carefully. Access Key label here This kit contains: SECURITY SEAL Paper Cup Sample Tube Security
just between us Urine Collection Kit Before attempting to collect your sample, please read these instructions carefully. Access Key label here This kit contains: SECURITY SEAL Paper Cup Sample Tube Security
Safe Handling and Disposal of Sharps
 SBC Children, Families And Community Health Service Statement of Intent Safe Handling and Disposal of Sharps To provide clear guidelines for the safe handling and disposal of all sharps in order that the
SBC Children, Families And Community Health Service Statement of Intent Safe Handling and Disposal of Sharps To provide clear guidelines for the safe handling and disposal of all sharps in order that the
UPEI Waste Disposal Protocol
 UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
BODY ART TEMPORARY EVENT SPONSOR APPLICATION PACKET
 BODY ART TEMPORARY EVENT SPONSOR APPLICATION PACKET Attached are instructions for event sponsors and body artist participants. The information should be read carefully. The sponsor must work with the Kern
BODY ART TEMPORARY EVENT SPONSOR APPLICATION PACKET Attached are instructions for event sponsors and body artist participants. The information should be read carefully. The sponsor must work with the Kern
BODY ART ESTABLISHMENT PLANNING APPLICATION
 BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
Introductory Chemistry
 Introductory Chemistry Lab 1: Introduction and Safety Objectives Learn how work to safely in the chemical laboratory Learn when and how to use the safety equipment in the chemical laboratory Learn the
Introductory Chemistry Lab 1: Introduction and Safety Objectives Learn how work to safely in the chemical laboratory Learn when and how to use the safety equipment in the chemical laboratory Learn the
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS
 PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
What is infection control?
 Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Welcome to the Hazard Communication Course
 Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Standard Operating Procedure for disposal of biological waste
 Standard Operating Procedure for disposal of biological waste Effective date: 22 nd March 2018 Review due date: 19 th March 2018 Original Author Name: Rebecca Toone Position: Technician Date: 29.07.2013
Standard Operating Procedure for disposal of biological waste Effective date: 22 nd March 2018 Review due date: 19 th March 2018 Original Author Name: Rebecca Toone Position: Technician Date: 29.07.2013
#74 - CHANGING A MOIST TO DRY DRESSING (TEST)
 #74 - CHANGING A MOIST TO DRY DRESSING (TEST) I acknowledge I have physically practiced and successfully learned the following skill(s): Student: Date: TIME LIMIT:30 Minutes TEST INCLUDES SKILLS FROM #56,
#74 - CHANGING A MOIST TO DRY DRESSING (TEST) I acknowledge I have physically practiced and successfully learned the following skill(s): Student: Date: TIME LIMIT:30 Minutes TEST INCLUDES SKILLS FROM #56,
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
Material Safety Data Sheet
 44 BRADY STREET P.O.BOX 52 VIRGINIA South Australia 5120 PH: (08) 8380 9976 FAX: (08) 83809977 24 HR: 0418 825 300 Material Safety Data Sheet Issue Date:- 2 nd June 2010 Page 1 of 5 1. IDENTIFICATION OF
44 BRADY STREET P.O.BOX 52 VIRGINIA South Australia 5120 PH: (08) 8380 9976 FAX: (08) 83809977 24 HR: 0418 825 300 Material Safety Data Sheet Issue Date:- 2 nd June 2010 Page 1 of 5 1. IDENTIFICATION OF
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control
 The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control Author University Health Service, The University of Hong Kong Approved By Task Force on Infectious Diseases,
The University of Hong Kong Recommendations on Cleaning / Disinfection for Infection Control Author University Health Service, The University of Hong Kong Approved By Task Force on Infectious Diseases,
PREPARATION OF BLOOD FILMS FOR MALARIA DETECTION
 PREPARATION OF BLOOD FILMS FOR MALARIA DETECTION Materials for Preparation of Malaria Smears: Clean and wrapped slides Sterile lancets 70% ethanol and water Absorbent cotton wool Surgical gloves Lint-free
PREPARATION OF BLOOD FILMS FOR MALARIA DETECTION Materials for Preparation of Malaria Smears: Clean and wrapped slides Sterile lancets 70% ethanol and water Absorbent cotton wool Surgical gloves Lint-free
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
APPROVAL REVIEW PROCEDURES
 Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Service to be provided is verified with the client and/or other operators. procedures and relevant legislation
 Prepare clients for salon services This unit describes the skills and knowledge required to prepare clients for a range of salon services. This unit of competency may apply to a range of roles in the workplace
Prepare clients for salon services This unit describes the skills and knowledge required to prepare clients for a range of salon services. This unit of competency may apply to a range of roles in the workplace
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
Student Performance Guide. Student Performance Guide. Student Performance Guide. Student Performance Guide. LESSON 3-3 Bleeding Time
 LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
Safe Handling and Disposal of Sharps. Reference Guide
 Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps
 Statement of Intent Safe Handling and Disposal of Sharps To provide clear guidelines for the safe handling and disposal of all sharps in order that the risk of inoculation injury and transmission of blood
Statement of Intent Safe Handling and Disposal of Sharps To provide clear guidelines for the safe handling and disposal of all sharps in order that the risk of inoculation injury and transmission of blood
COLLECTION INSTRUCTIONS - SAMPLES (VARIOUS TYPES)
 COLLECTION INSTRUCTIONS - SAMPLES (VARIOUS TYPES) You need to complete the necessary forms for each sample collected. To avoid any possible contamination, please ensure that gloves are worn during the
COLLECTION INSTRUCTIONS - SAMPLES (VARIOUS TYPES) You need to complete the necessary forms for each sample collected. To avoid any possible contamination, please ensure that gloves are worn during the
BTB000 Manufacturers Product Code: TAB/R/DR
 BTB000 Manufacturers Product Code: TAB/R/DR Red Polythene Drug Round Tabard (21 x 24 / 30mu) black print Drug Round in Progress. One size fits all. Drug round tabards improve the efficiency and accuracy
BTB000 Manufacturers Product Code: TAB/R/DR Red Polythene Drug Round Tabard (21 x 24 / 30mu) black print Drug Round in Progress. One size fits all. Drug round tabards improve the efficiency and accuracy
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
