Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
|
|
|
- Phebe Dorsey
- 5 years ago
- Views:
Transcription
1 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel Deborah Jung Microbiology Preparation Technician Shannon McNew Instructor of Biology, Scientific Equipment Coordinator for the Regional Campuses Equipment Coolers The micro tech. will send labeled coolers (usually 2) to each regional campus with microbiological media and live specimen cultures according to the laboratory schedule. It is the responsibility of the instructor to promptly unpack the cooler, store the media/cultures appropriately and send the empty cooler back to the Cape campus. Equivalently, the tech will unpack and process materials sent to the Cape campus then return the empty cooler back to its respective campus. We would like to keep one cooler at each campus for shipping waste to Cape campus. The coolers are transported daily by a courier. The courier service is managed by Facilities Management. The micro tech or the instructor will place the cooler(s) in the office or designated area for pick up by the courier. Our courier is David Hinkle dhinkle@semo.edu. See schedule details in the Office of Extended Learning Faculty Manual available on the Office of Extended Learning webpage. Sending media and cultures to regional campuses in coolers For packing and securing purposes, foam or other packing material may be used but must be covered. Foam pieces should be placed in self seal plastic bags (Ziplocks) and changed occasionally. Sterile media should be shipped in a separate cooler from cultures or contaminated waste. All liquid cultures should be transported in screw-top tubes placed in test tube racks. The racks of tubes should be double bagged in clear Infecon biohazard bags. Racks should then be placed in cooler and secured for transport with foam packing material. Use 3-4 strips of wide masking tape to 1
2 secure cooler lid. Write on masking tape name of instructor and destination. This is to help prevent spills and expedite transport. Sending waste and unused media to Cape campus in coolers Used tubes and all liquid cultures should be placed back in test tube racks, double bagged with clear Infecon biohazard bags. Other waste to be autoclaved (plates, gloves, etc ) should be double bagged in orange autoclave/biohazard bags with open end tied or secured. These bags will NOT be inspected but go directly into autoclave for sterilization. At end of semester, send back any unused media in separate cooler. Cooler contents should be secured with bagged packing foam. Use 3-4 strips of wide masking tape to secure cooler lid. Write on masking tape name (Deb. Jung) and destination (Cape Micro Lab RH313). This is to help prevent spills and expedite transport. The microbiology prep tech will disinfect all coolers at least once a semester or more often as needed. Biohazard and Infecon Transport Bags All waste and specimen cultures should be double bagged for transport. As you run low on bags, please add to the yearly Big Order or contact S. McNew. Petri Plate and Glove Disposal Bin Mid-sized (about 5 gal.) plastic biohazard containers with lids are used for petri plate and glove disposal. Line the container with orange biohazard bag. It is important to encourage students to use aseptic technique when handling the container lids. When container gets ¾ full or at the end of the semester, remove biohazard bag, double bag it, then secure open ends. Ship it to Cape campus in cooler to be autoclaved. These may contain gloves from microbiology or anatomy & physiology laboratory activities. 2
3 Test Tube Use and Disposal Baskets may be used on site for storage of tubed microbiological media. When test tubes have been used, place them in the test tube racks. Double bag the racks in clear Infecon bags and place them upright inside a cooler for transport back to the Cape campus. The micro tech will autoclave and wash for re-use. NOTE: Test tube labels or tape MUST be removed prior to transport. Pipette Disposal Lidded cylinders that sit on the floor (about 2ft. tall) are for pipette disposal. Line the cylinder with an orange biohazard bag. When full or at the end of the semester, double bag, secure, and ship back to Cape campus in a cooler. Be careful with broken pipettes; place bags with broken pipettes in a small cardboard box before placing in cooler. Be sure to label box appropriately. Sharps Bins Used microscope slides, small broken glass pieces, scalpel blades, and other sharp objects should be disposed of in the small sharps containers. When full, close lid until it clicks. Double bag it in clear Infecon bags, secure in a cooler for transport to Cape campus. Be sure to order a replacement container as the full ones are autoclaved and destroyed. 3
4 Broken Glass Box This box is for large pieces of uncontaminated broken glass only. It is best to keep the box in the prep room away from student view. They tend to fill them with paper towels and other non-glass waste. Small glass pieces can be discarded in sharps bin. When the box is full or full enough, tape the lid closed (both the hole in the top AND around the lid and place it into the dumpster. Compound Microscopes Please make sure your students always use two hands to carry the microscopes (and dissecting scopes). Microscopes should be placed on table and picked up to be moved, avoid scooting or sliding the microscopes across the table surface, vibrations will destroy the alignment of the lenses. Microscopes are expensive and cost about $3,000 to replace. Immersion oil should only come into contact with the 100x objective lens. If other objective lenses get oil on them, it will destroy them. ALL oil should be removed from the microscopes, cords bundled, and switched off prior to storage back in cabinet. Dissecting Microscopes For the dissecting microscopes, please follow similar guidelines (above) for the compound microscopes. All should be free of dust and debris before being stored. Clearly label scope if it develops a problem. 4
5 Other Equipment and supplies you will find in the biology teaching laboratory: Lens Paper Lens paper should be used for the scopes. Instruct students to use the lens paper while cleaning scopes. Use lens paper only on the scopes, not paper towels. Paper towels will scratch lenses. Dispose of in regular trash. Incubator When the incubator is in use, a daily temperature log sheet is suggested to track the temperature/function of the incubator. Place a thermometer inside the incubator around mid-level (some incubators already have small incubator thermometers inside). Please label, date, and initial each temperature log and keep in folder attached to incubator. Temperature logs are important so that we know how the incubator is functioning. Please report any temperature problems to Shannon McNew. Empty, clean, and turn off incubator when not in use. Do not store other equipment or supplies on top of incubator. Fume Hood The fume hood may be used for any science class but most often used by the chemistry classes. While in use, please pull sash and keep free of litter and debris. When not in use, fume hoods should be turned off if allowed. 5
6 Centrifuge Please keep centrifuge free of dust and debris. Unplug when not in use. Clean, dry, and re-store the centrifuge tubes after use. See manual for operation instructions. Spectrophotometer Please keep spectrophotometer free of dust and debris. Replace cover and unplug when not in use. Clean, dry, and re-store the spec tubes after use. Digital Scale All scales should be secured to the wall or bench. Scales tend to be the first piece of scientific equipment stolen from classrooms. Always keep free of dust and chemicals. Use brush or soft cloth to clean. 6
7 Light Box Water Bath Wash Bottles For distilled water or a 10% bleach solution. Please label all bottles while they are in use. After each semester, bottles should be drained of contents, cleaned, dried, and put away. 7
8 Microscope Slide Racks Slide racks are used by some Microbiology instructors for staining bacteria slides. After use, wipe racks clean with an alcohol soaked paper towel. All stain should be removed from racks prior to storage. Reagent Bottles Reagent bottles should be kept full for student use. Extra reagent is kept in the prep room. Microbiological stains and reagent supply should be ordered for each campus with the yearly Big Order. A small amount may be requested from the micro tech as needed. All bottles containing stain or reagents must be clearly labeled. All reagent bottles while not in use should be kept in the prep room. Live microbiological specimen cultures Live bacteria cultures will be sent to the regional campuses via courier for use by the Microbiology classes. For safety compliance, plated or slant cultures will be sent whenever possible. Upon arrival, cultures should be placed in the prep room refrigerator until ready for use in the classroom. While most of the microorganisms used in the classroom have minimal potential hazard to students and environment, standard safety practices/techniques must be used. Please read the copy of Standard Safety Practices in the Microbiology Laboratory in the appendix of this document for more information. You may use it as a guide to help you establish good laboratory procedures in the classroom. You may also reference the Guidelines for Biosafety in Teaching Laboratories manual that is located on the counter in the prep room for more information. Preserved Specimen Dissection Waste Throughout the semester, dissection waste generated from Anatomy & Physiology or other biology laboratory activities should be kept bagged (regular black trash bags are O.K.) and covered in the prep room. At the end of the semester or as needed, double bag the waste, pack it in a cooler, and send it to the Cape campus via the courier. Use 3-4 strips of wide masking tape to secure cooler lid. Please label with Dissection Waste and address it to Shannon McNew RH224. Dissection waste will be incinerated. It is important that no preservation fluid is included in the bags. All used preservation fluid that is no longer needed can remain in the buckets. Secure the lid on the bucket, label the bucket for disposal and contact Shannon McNew or Autumn Gentry, Safety Specialist agentry@semo.edu It will be picked up by Autumn Gentry or her staff in the next chemical waste haul. 8
9 Appendix Standard Safety Practices in the Microbiology Laboratory Laboratorians working with infectious agents are subject to laboratory acquired infections through accidents or unrecognized incidents. The degree of hazard depends upon the virulence of the biological agent concerned and host resistance. Laboratory-acquired infections occur when microorganisms are inadvertently ingested, inhaled, or introduced into the tissues. The primary laboratory hazard associated with enteric pathogens such as Shigella and E. coli O157:H7 is accidental ingestion. Biosafety Level 2 (BSL-2) practices are suitable for work involving these agents, which are a moderate potential hazard to personnel and the environment. BSL-2 requirements: Laboratory personnel have specific training in handling pathogenic agents and are directed by competent scientists; Access to the laboratory is limited when work is being conducted; Extreme precautions are taken with contaminated sharp items; Certain procedures in which infectious aerosols or splashes may be created are conducted using protective clothing and equipment. A. Standard Microbiological Safety Practices The safety guidelines listed below apply to all microbiology laboratories regardless of biosafety level. Limiting access to laboratory Biohazard signs or stickers should be posted near all laboratory doors and on all equipment (incubators, hoods, refrigerators, freezers) used for laboratory work. Children under 12 years of age and pets are not allowed in laboratory areas. All laboratories should be locked when not in use. All freezers and refrigerators located in corridors should be locked. Handwashing Each laboratory should contain a sink for handwashing. Frequent handwashing is one of the most effective procedures for avoiding laboratoryacquired infections. Hands should be washed with an appropriate germicidal soap before exiting the laboratory or after handling infectious materials. Eating Eating, drinking, and smoking are not permitted in the work areas. Food must be stored and eaten outside of the work area in designated areas used for that purpose only. Do not lay personal articles such as handbags or eyeglasses on the workstations. Gloves Regardless of the type of infectious material, gloves should be worn when performing potentially hazardous procedures (e.g., slide agglutination) in which there is a risk of splashing or skin contamination or when the laboratory worker has cuts or broken skin on his or her hands. Gloves should always be worn when 9
10 handling clinical specimens, body fluids, and tissues from humans and animals. These tissues should be assumed to be positive for hepatitis B virus, HIV, other bloodborne pathogens, or Mycobacterium tuberculosis. Gloves must be removed when contaminated by splashing or spills or when work with infectious materials is completed. Gloves should not be worn outside the laboratory. Do not use the telephone or open doors with gloves that have been used in laboratory procedures. Mouth pipetting Mouth pipetting should be strictly prohibited in the laboratory. Rubber bulbs or mechanical devices should be used. Sharps A high degree of precaution must always be taken with any contaminated sharp items, including needles and syringes, slides, pipettes, capillary tubes, and scalpels. Dispose of sharps in designated containers. To minimize finger sticks, used disposable needles must not be bent, sheared, broken, recapped, removed from disposable syringes, or otherwise manipulated by hand before disposal. Nondisposable sharps, including syringes, should be placed in a labeled discard pan for decontamination before cleaning. Broken glassware should not be handled directly by hand but should be removed by mechanical means such as a brush and dustpan, tongs, or forceps. Aerosols Perform all procedures carefully to minimize the creation of splashes or aerosols. Techniques that tend to produce aerosols should be avoided. Cool inoculating wires and loops by holding them still in the air for 5 to 10 seconds before touching colonies or clinical material. Loops containing infectious material should be dried in the hot air above the burner before flaming. Vortexing and centrifugation should be done in closed containers. Gauze should be used to remove the tops on blood specimens and should be placed around the top of blood culture bottles to minimize aerosol production during removal of the needle. Needles should never be cut or removed from the syringe before autoclaving. All body fluids should be centrifuged in carriers with safety caps only. When procedures with a high potential for creating infectious aerosols are conducted or when there is a risk of splashing or spraying the face with infectious or other hazardous materials, laboratory work should be conducted in a safety cabinet or with face protection (goggles, mask, face shield or other splatter guards). Procedures that pose a risk may include centrifuging, grinding, blending, vigorous shaking or mixing, sonic disruption, opening containers of infectious materials whose internal pressures may be different from ambient pressures, inoculating animals intranasally, and harvesting infected tissues from animals or eggs. Face protection should also be used when working with high concentrations or large volumes of infectious agents. Decontaminating bench tops and other surfaces Bench tops should be wiped with a disinfectant (a phenolic disinfectant, 1% sodium hypochlorite, or 70% alcohol) routinely after working with infectious agents or clinical specimens or after spills, splashes, or other contamination by infectious materials. Solutions of disinfectants should be maintained at the work station (see Disinfectants below). 10
11 Disposal of contaminated materials All discarded plates, tubes, clinical samples or other contaminated materials are to be placed in disposal containers at each bench. Special disposal boxes must be used for sharps such as syringes or broken glass to minimize the risk of injury. Avoid overfilling such containers. Containers of contaminated material should be carefully transported to the autoclave room and autoclaved before disposal. Autoclaving An autoclave must be available for the BSL-2/3 laboratory and must be operated only by personnel who have been properly trained in its use. To verify that each autoclave is working properly, spore strips or other biological indicators designed to test for efficiency of sterilization should be included in autoclave loads on a regular basis. Each autoclave load should be monitored with temperaturesensitive tape, thermograph, or other means (e.g., biological indicators). General laboratory policies All areas of the laboratory must be kept clean and orderly. Dirt, dust, crowding, or clutter is a safety hazard and is not consistent with acceptable biological research. Floors should be kept clean and free of unnecessary clutter. They should be washed with a germicidal solution on a regular basis and after any spills of infectious material have occurred. Refrigerators and freezers Refrigerators and freezers should be regularly inspected for the presence of broken vials or tubes containing infectious agents. Wear gloves and proper attire when removing and discarding broken material. Refrigerators and freezers should be regularly cleaned with a disinfectant and defrosted to prevent possible contamination and temperature failure. Fire prevention Keep burners away from lamps and flammable materials. Bulk flammable material must be stored in the safety cabinet. Small amounts of these materials, such as ethyl acetate, ethyl alcohol, and methanol, can be stored in safety containers. Turn off burners when not in use. Know the location of fire extinguishers, fire blankets, and showers. Fire safety instructions and evacuation routes should be posted. B. Special Practices Transport of biohazardous materials Transport of biohazardous materials from one building to another increases the risk of breakage and spills. If transport is necessary, the primary infectious agent container (regardless of size) must be placed in an unbreakable second container that can be sealed (e.g., screw-top tube, plastic bag). Disinfectants Organisms may have different susceptibilities to various disinfectants. As a surface disinfectant, 70% alcohol is generally effective for the Enterobacteriaceae, 11
12 but other organisms are more resistant. However, 70% alcohol is not the disinfectant of choice for decontaminating spills. Phenolic disinfectants, although expensive, are usually effective against many organisms. Always read disinfectant labels for manufacturers recommendations for dilution and for exposure times for efficacy, especially before use on BSL-3 organisms such as Mycobacterium tuberculosis. A good general disinfectant is a 1:100 (1%) dilution of household bleach in water; at this dilution, bleach can be used for wiping surfaces of benches, hoods and other equipment. A 1:10 (10%) dilution of bleach is corrosive and will pit stainless steel and should not be used routinely; however, it may be used to clean up spills of cultured or concentrated infectious material where heavy contamination has occurred. Dilutions of sodium hypochlorite should be made daily from a stock solution. Decontamination of spills The following procedure is recommended for decontaminating spills. Isolate the area to prevent anyone from entering. Wear gloves and protective clothing (gown or lab coat; mask if the spill may contain a respiratory agent or if the agent is unknown). Absorb or cover the spill with disposable towels. Saturate the towels with an appropriately diluted intermediate or high level disinfectant (e.g., a phenolic formulation or household bleach). Place disinfectant-soaked towels over the area and leave them in place for at least 15 minutes before removing and discarding them. Wipe area using clean disinfectant-soaked towels and allow area to air dry. Place all disposable materials used to decontaminate the spill into a biohazard container. Handle the material in the same manner as other infectious waste. Accidents All injuries or unusual incidents should be reported immediately to the supervisor. When cuts or puncture wounds from potentially infected needles or glassware occur, the affected area should be promptly washed with disinfectant soap and water. In the event of a centrifuge accident in which safety carriers have not been used, other personnel in the area should be warned immediately and the area isolated to prevent anyone from entering. C. Protective Clothing and Equipment Laboratory coats Protective coats, gowns, smocks, or uniforms designated for laboratory use must be worn while working in the laboratory. This protective clothing should be removed and left in the laboratory before leaving for non-laboratory areas. All protective clothing is either disposed of in the laboratory or laundered by the institution; it should never be taken home by personnel. Gloves Regardless of the type of infectious material, gloves should be worn when performing potentially hazardous procedures (e.g., slide agglutination) in which there is a risk of splashing or skin contamination or when the laboratory worker has cuts or broken skin on his or her hands. Gloves should always be worn when handling clinical specimens, body fluids, and tissues from humans and animals. These tissues should be assumed to be positive for hepatitis B virus, HIV, other 12
13 bloodborne pathogens, or Mycobacterium tuberculosis. Gloves must be removed when contaminated by splashing or spills or when work with infectious materials is completed. Gloves should not be worn outside the laboratory. Do not use the telephone or open doors with gloves that have been used in laboratory procedures. Dispose of all used gloves by discarding them with other disposable materials and autoclaving. Hands should be washed immediately after removing gloves. Barrier precautions Clinical specimens, body fluids, and tissues from humans and animals should be assumed to be positive for hepatitis B virus, HIV, other bloodborne pathogens, or Mycobacterium tuberculosis. These materials should be handled in a safety cabinet or using other barrier precautions such as goggles, mask, face shield or other splatter guards whenever there is a potential for creating an aerosol. References Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in microbiological and biomedical laboratories. Washington, DC: U.S. Government Printing Office; 1999: stock no World Health Organization. Laboratory biosafety manual, 2nd edition. Geneva: WHO; 1993: ISBN Standard Safety Practices in the Microbiology Laboratory 13
PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015
 PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015 Personnel Maija Bluma (573-651-2069; mbluma@semo.edu) is the Microbiology Preparation
PROTOCOL FOR SCIENCE EQUIPMENT USAGE WITH EMPHASIS ON BS240/242: MICROORGANISMS AND THEIR HUMAN HOSTS Updated July 2015 Personnel Maija Bluma (573-651-2069; mbluma@semo.edu) is the Microbiology Preparation
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
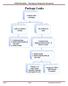 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Introductory Chemistry
 Introductory Chemistry Lab 1: Introduction and Safety Objectives Learn how work to safely in the chemical laboratory Learn when and how to use the safety equipment in the chemical laboratory Learn the
Introductory Chemistry Lab 1: Introduction and Safety Objectives Learn how work to safely in the chemical laboratory Learn when and how to use the safety equipment in the chemical laboratory Learn the
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
UPEI Waste Disposal Protocol
 UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Acid Or Alkali? Testing With Cabbage
 Acid Or Alkali? Testing With Cabbage Topic Using vegetables as an acid/base indicator Introduction Forensic scientists need to discover if someone has tampered with liquids (e.g., cosmetics, cleaning products,
Acid Or Alkali? Testing With Cabbage Topic Using vegetables as an acid/base indicator Introduction Forensic scientists need to discover if someone has tampered with liquids (e.g., cosmetics, cleaning products,
Chemistry Lab Safety Handout PSI Chemistry
 Chemistry Lab Safety Handout PSI Chemistry Name Chemistry is exciting! Each day in the laboratory you are given the opportunity to confront the unknown and to understand it. You can wonder how things work,
Chemistry Lab Safety Handout PSI Chemistry Name Chemistry is exciting! Each day in the laboratory you are given the opportunity to confront the unknown and to understand it. You can wonder how things work,
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
GENERAL CHEMISTRY LABORATORY SAFETY
 GENERAL CHEMISTRY LABORATORY SAFETY 1. Read the experiment before coming to Keyes 405. The more prepared you are, the safer and more efficient you will be in lab. 2. Think about what you need to wear to
GENERAL CHEMISTRY LABORATORY SAFETY 1. Read the experiment before coming to Keyes 405. The more prepared you are, the safer and more efficient you will be in lab. 2. Think about what you need to wear to
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
Safe Handling and Disposal of Sharps. Reference Guide
 Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Student Performance Guide. Student Performance Guide. Student Performance Guide
 LESSON 8-2 Collecting and Processing Specimens for Parasite Examination Student Performance Guide LESSON 8-3 Microscopic Methods for Student Performance Guide LESSON 8-4 Preparing and Staining Smears for
LESSON 8-2 Collecting and Processing Specimens for Parasite Examination Student Performance Guide LESSON 8-3 Microscopic Methods for Student Performance Guide LESSON 8-4 Preparing and Staining Smears for
2.6-1 SCIENCE EXPERIMENTS ON FILE Revised Edition. Cloud Chamber
 2.6-1 SCIENCE EXPERIMENTS ON FILE Revised Edition Cloud Chamber Topic Cloud formation Time 1 hour! Safety Please click on the safety icon to view safety precautions. Do not touch dry ice with bare hands.
2.6-1 SCIENCE EXPERIMENTS ON FILE Revised Edition Cloud Chamber Topic Cloud formation Time 1 hour! Safety Please click on the safety icon to view safety precautions. Do not touch dry ice with bare hands.
Standard Operating Procedure for disposal of biological waste
 Standard Operating Procedure for disposal of biological waste Effective date: 22 nd March 2018 Review due date: 19 th March 2018 Original Author Name: Rebecca Toone Position: Technician Date: 29.07.2013
Standard Operating Procedure for disposal of biological waste Effective date: 22 nd March 2018 Review due date: 19 th March 2018 Original Author Name: Rebecca Toone Position: Technician Date: 29.07.2013
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
Name: Date: Class: Safety First!
 Safety First! Creating, exploring, inventing, investigating these activities are essential to the study of science, and they are why working in a creative laboratory environment is so important. To make
Safety First! Creating, exploring, inventing, investigating these activities are essential to the study of science, and they are why working in a creative laboratory environment is so important. To make
Spring 2005 Pollution Prevention Workshop For Healthcare
 Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Original Date:
 Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
Policy: C-08-PRO Reviewed: 7/08
 Written: 1979 Policy: Reviewed: 7/08 Procedure Policy Manual Revised: 81, 84, 85, 86, 87, 88, 89, 90, 92, 93, 95 Ambulatory Care Division 2/97, 7/98, 7/00, 7/02, 7/04, 7/06, 2/09, 7/10 LSU Health Sciences
Written: 1979 Policy: Reviewed: 7/08 Procedure Policy Manual Revised: 81, 84, 85, 86, 87, 88, 89, 90, 92, 93, 95 Ambulatory Care Division 2/97, 7/98, 7/00, 7/02, 7/04, 7/06, 2/09, 7/10 LSU Health Sciences
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions
 Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
Section 4 Procedures for Biohazard Control
 Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Lenis Needle-free Safety Syringe Device User Manual
 Lenis Needle-free Safety Syringe Device User Manual 1 Table of Contents Welcome.3 Lenis Kit Components.4 Instructions 5-9 Maintenance and Care..10 Troubleshooting. 11 Warranty.12 Precautions 13 Return
Lenis Needle-free Safety Syringe Device User Manual 1 Table of Contents Welcome.3 Lenis Kit Components.4 Instructions 5-9 Maintenance and Care..10 Troubleshooting. 11 Warranty.12 Precautions 13 Return
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Instructor Guide. Georgia Department of Technical and Adult Education. Decontamination and Infection Control
 Instructor Guide Georgia Department of Technical and Adult Education Decontamination and Infection Control Copyright October 2002 by. All rights reserved. No part of this manual may be reproduced or transmitted
Instructor Guide Georgia Department of Technical and Adult Education Decontamination and Infection Control Copyright October 2002 by. All rights reserved. No part of this manual may be reproduced or transmitted
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System. Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe
 Chapter 6 Phlebotomy Procedure 29 Performing A Venipuncture Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe Procedure
Chapter 6 Phlebotomy Procedure 29 Performing A Venipuncture Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe Procedure
