US A United States Patent Patent Number: 5,643,584 Faring et al. 45 Date of Patent: Jul. 1, 1997
|
|
|
- Mabel Ryan
- 5 years ago
- Views:
Transcription
1 IIIHIII US A United States Patent Patent Number: Faring et al. Date of Patent: Jul. 1, 1997 (54) AQUEOUS GEL RETINOID DOSAGE FORM 56) References Cited (75. Inventors: Richard K. Farng, East Brunswick; U.S. PATENT DOCUMENTS Gerard J. Gendimenico, Neshanic 3,729,568 4/1973 Kligman 424/318 Station.James A. Meiss, East 4,826,871 5/1989 Gressel ,438 Brunswick, Shirley M. Ng, 4,966,773 /1990 Gressel /489 Bridgewater Stanley B. Wrobel, Jr., 5,476,852 12/1995 Cauwenbergh... 54/2 Middlesex, all of N.J. (73) Assignee: Ortho Pharmaceutical Corporation, FOREIGN P. DOC S Raritan, N.J. 90/ /1990 WIPO. 90/ /1990 WIPO 21 Appl. No.: 444,1 22 Filled: May 18, 1995 Primary Examiner-Sallie M. Gardner 4) ) 57 ABSTRACT Related U.S. Application Data This invention relates to aqueous gel retinoid compositions (63) Continuation of Ser. No. 132,014, Oct 5, 1993, abandoned, and their methods of use. More specifically, the aqueous gel Which is a continuation of Ser. No. 869,684, Apr. 16, 1992, retinoid compositions of the invention utilize micronized abandoned. particles of retinoids, particularly tretinoin, to provide an (51 int. Cl.... A61K 7/00; A61K 7/48 aqueous gel for topical application of such retinoids to the 52 U.S. Cl /401; 514/994; 514/947; skin which minimizes skin irritation while retaining drug 514/975; 514/859 efficacy. 58 Field of Search /401; 514/844, 514/947, 847,944, 975, 859; 568/ Claims, No Drawings
2 1. AQUEOUS GEL RETINOID DOSAGE FORM This is a continuation, of application Ser. No. 08/132, 014, filed Oct. 5, 1993, now abandoned, which is a 1.60 continuation of Ser. No. 07/869,684, now abandoned, filed Apr. 16, 1992, which are hereby incorporated by reference. FIELD OF THE INVENTION This invention relates to aqueous gel vehicles for retin oids. More specifically, micronized particles of retinoids, particularly tretinoin, are incorporated into aqueous gel vehicles to provide a gel composition for topical application of such retinoids to the skin. BACKGROUND OF THE INVENTION Retinoids (e.g. Vitamin A and its derivatives) are sub stances which are known to have a broad spectrum of biological activity. More specifically, these substances affect cell growth, differentiation and proliferation. Retinoids affect the differentiation, maintenance, and proliferation of many types of cells, whether they are of ectodermal, endo dermal or mesodermal origin. Retinoids have found clinical utility in the treatment of acne vulgaris, severe cystic acne, psoriasis, and other disorders of keratinization. Possible uses of retinoids are being explored in the prophylaxis and treatment of cancer. See generally, Pawson, B. A. et al., "Retinoids at the Threshold: Their Biological Significance and Therapeutic Potential", Journal of Medicinal Chemistry : (1982). It is known to use certain retinoids, particularly tretinoin, topically for treatment of acne as set forth in U.S. Pat. No. 3,729,568. Other known topical uses of tretinoin include, in addition to ache treatment, treatment of senile comedones, nevus comedonicus, linear verrucous nevus, plantar warts, pseudofolliculitis, keratoacanthoma, solar keratosis of extremities, callosities, keratosis palmaris et plantaris, Dari er's disease, ichthyosis, psoriasis, acanthosis nigricans, lichen planus, molluscum contagiosum, reactive perforating collagenosis, melasma, corneal epithelial abrasion, geo graphic tongue, Fox-Fordyce disease, cutaneous metastatic melanoma and keloids or hypertrophic scars see, e.g., Thomas, J. R., et al., The Therapeutic uses of Topical Vitamin AAcid', Journal of American Academy of Derma tology 4:5-516 (1981). U.S. Pat. No. 4,603,146 discloses methods for treating Sundamaged human skin topically with tretinoinin an emol lient vehicle. U.S. Pat. Nos. 4,877,805 and 4,883,342 dis close methods for the treatment of Sundamaged human skin using retinoids. U.S. Pat. No. 5,051,449 discloses treatment of cellulite with retinoids. The above-noted patents disclose formulations of retin oids in various moisturizing bases such as creams or oint ments. Retinoids, such as tretinoin, in cream formulations, may meet the needs of certain individuals but may be found undesirable by other individuals. It is also suggested in European Patent Application No to Maxam, Inc. that volatile vehicles, such as alcohols, which may dry or otherwise harm the skin should be avoided. Maxam's patent application discloses an aqueous gel formulation of tretinoin which utilizes a glycerin and a proteinaceous material, e.g. soluble animal collagen, to stabilize its gelling agent. The present invention does not require the use of glycerin nor a proteinaceous material to stabilize its gel formulation. SUMMARY OF THE INVENTON It is therefore an object of the present invention to provide a retinoid aqueous gel composition for topical administra 40 2 tion of retinoid to the skin which minimizes skin irritation but maintains a high degree of therapeutic effectiveness. As embodied and fully described herein, the present invention provides a retinoid aqueous gel composition for therapeutic topical administration of retinoid to the skin comprising a therapeutically effective amount of micronized retinoid particles; a surfactant in an amount effective to enhance penetration of retinoid into the skin; a preservative; a gelling agent composition in an amount sufficient to provide body to the aqueous gel; and water qs to 0%. In preferred embodiments the composition comprises in weight by total weight of the composition: to 0.5% micron ized retinoid particles; to 1.0% surfactant; to 2.0% preservative; 0.01% to 0.3%. antioxidant; 1.0% to 2.0% gelling agent; sufficient base to attain a ph in the range of 4.0 to 7.0; and water qs to 0%. In preferred embodiments of the invention, the retinoid is tretinoin. In other preferred embodiments, the micronized particles comprise at least 90% of the particles in a size range of from 1 to 40 microns, more preferably from 1 to microns, and most preferably with a mean size in the range of 1 to microns. In preferred embodiments the surfactant of the aqueous gel composition is selected from the group consisting of octoxynol, polyethylene glycol glyceryl stear ate and monoxynol. In preferred embodiments the preserva tive is selected from the group consisting of benzyl alcohol, sorbic acid, pardbens, imidazolidinyl urea (imidurea) and combinations thereof, more preferably, a combination of benzyl alcohol and sorbic acid. In preferred embodiments of the composition, the gelling agent is selected from the group consisting of acrylic acid crosslinked with the allyl ether of sucrose or pentaerythritol; and poloxamer. Preferably, the gelling agent is an acrylic acid polymer known to the art as carbomer. In preferred embodiments of the invention, the base used to raise the ph of the composition to a ph of between 4.0 to 7.0 is sodium hydroxide or triethanolamine, preferably, sodium hydroxide which is added in an amount of about 0.1% to 0.6% to provide a ph to the composition of between 4.5 to 5.5. In preferred embodiments of the invention the antioxidant is butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), alpha-tocopherol, or ascorbic acid. In preferred embodiments of the invention the aqueous gel composition comprises from about % to 0.5% retinoid, preferably, 0.01% to 0.2% retinoid. In a preferred embodiment of the invention the aqueous gel composition does not contain any water soluble lower alkyl alcohol. In other embodiments of the aqueous gel of the invention polyvinylpyrrollidone is added thereto to inhibit crystal growth of the micronized retinoids. In further preferred embodiments a chelating agent is added thereto. Methods of using the aqueous gel composition of the invention are provided to increase the therapeutic effective ness of retinoids for topical application to the skin compris ing the step of delivering a dispersion of micronized retinoid particles in an aqueous gel vehicle to the intended site of skin application. In preferred embodiments of the invention the method provides for delivering micronized retinoid to an intended site of application using an aqueous gel vehicle which has an alcohol content of less than 2% by weight of the total weight of the composition, more preferably the aqueous gel vehicle contains no water soluble lower alkyl alcohol. In other embodiments of the invention a method is provided for reducing the irritation associated with retinoid therapy which comprises the steps of incorporating micron
3 3 ized retinoid in an aqueous gel vehicle and applying the micronized retinoid aqueous gel composition to the skin of a patient. In preferred embodiments the aqueous gel com position used in this method comprises any of the preferred embodiments of the formulation described above. DETALED DESCRIPTION OF PREFERRED EMBODIMENTS OF THE INVENTION Reference will now be made in detail to preferred embodiments of the invention. Examples of the preferred embodiments are illustrated in the following Examples section. Retinoids have been defined narrowly as comprising vitamin A (retinol) and its derivatives, such as vitamin A aldehyde (retinal) and vitamin Aacid (tretinoin), which are metabolites of vitamin A. Subsequent research has, however, resulted in a larger class of chemical compounds that are termed retinoids because they have biological actions simi lar to vitamin A, even though there may be structural dissimilarities. Compounds useful in the present invention include all natural and/or synthetic analogs of vitamin A or retinol-like compounds which have similar therapeutic activities as demonstrated for example, by tretinoin. Accordingly, as used herein for purposes of the present invention, the term "retinoid will be understood to mean a natural or synthetic substance that elicits all or some of the biologic responses of retinoic acid or retinol by binding to and subsequently activating known and unknown cutaneous retinoic acid receptors. Also encompassed within the term "retinoid" for the purposes of the present invention, are stereoisomers of the retinoids, as well as pro-drugs thereof. Examples of retinoids include those disclosed in U.S. Pat. No. 4, , the entire disclosure of this patent is hereby incorporated herein by reference. The present invention provides an aqueous gel composi tion for topical administration of retinoids to the skin which increases the therapeutic effectiveness of such an application over alcoholic gel vehicles or oil-based vehicles while reducing the irritation that is associated with the applications of retinoids to the skin of certain sensitive patients. The aqueous gel composition of the invention also pro vides for increasing the safety of topically applied retinoid to the skin whereby micronized retinoids are provided to an intended site of application using an aqueous gel vehicle which preferably contains no lower alkyl alcohols. This elimination of lower alkyl alcohol reduces potential prob lems of drying the skin as well as reducing volatile fumes which may be undesirably inhaled or be flammable during production of alcohol gel products. The aqueous gel composition of the invention comprises at least one retinoid in accordance with the definition described above. In preferred embodiments of the invention the retinoid is tretinoin. The retinoid, preferably tretinoin, is provided in a micronized form whereby at least 90% of the retinoid particles are provided in the size range of 1 to 40 microns, more preferably 1 to microns. Most preferably, the micronized retinoid particles have a mean size in the range of 1 to microns. The retinoid particles which normally average above 40 microns to about 0 microns in size are reduced in particle size, i.e. micronized in accor dance with procedures known to the art such as using fluid-energy mills. The aqueous gel composition of the invention comprise in weight by total weight of the composition of from about to 0.5% micronized retinoid, preferably tretinoin, particles. In more preferred embodiments micronized tret O 60 4 inoin is present in the range of to 0.2%. This concen tration range is particularly advantageous for topical appli cation of retinoid and particularly tretinoin particles to the skin of a patient because it provides optimal effective amounts of retinoid to the skin for treatment of acne and/or treatment of sundamaged skin and other therapeutic appli cations. Using tretinoin as a standard for retinoids, tretinoin will typically be present in the composition of the invention in an amount of about to 0.5, preferably to 0.2% weight by total weight, and other more or less potent retinoids will be used in corresponding amounts equivalent thereto. Larger amounts of retinoid, e.g. tretinoin, present in the formulations may increase the chance for skin irritation. Amounts which are less than those provided in this range may not provide enough retinoid or tretinoin to provide effective treatment to the skin of a patient. The retinoid aqueous gel composition of the invention comprises from about 0.001% to 1.0% surfactant, weight by total weight of the composition. The surfactant is useful to enhance the penetration of the retinoid into the epidermis layer of the skin and also to disperse the retinoid particles throughout the aqueous media. This dispersion is important to provide consistent dosage forms and applications of the drugs for application of optimal treatments to the skin of patients. Surfactants which are useful in accordance with the invention include but are not limited to those selected from the group consisting of octoxynol (an anhydrous mixture of mono p-(1,1,3,3-tetra-methylbutyl)phenyl]ethers of poly ethylene glycols containing 5 to oxyethylene groups in the polyoxyethylene chain), polyethylene glycol glyceryl stearate and nonoxynol. Particularly preferred as a surfactant is octoxynol-13 (whereby an average of 13 oxyethylene groups are in the polyoxyethylene chain). The aqueous gel composition of the invention preferably comprises from about 0.005% to 2.0% preservative weight by total weight of the composition. The preservative is used to prevent spoilage of the aqueous gel during repeated patient use when it is exposed to contaminants in the environment from, for example, exposure to air or the patient's skin including contact with the fingers used for applying the therapeutic gel. Examples of preservatives useful in accordance with the invention include but are not limited to those selected from the group consisting of benzyl alcohol, sorbic acid, parabens, imidurea and combinations thereof. A particularly preferred preservative is a combina tion of about 0.5%-2.0% benzyl alcohol and 0.05% to 0.5% sorbic acid. The aqueous gel composition preferably includes an anti oxidant and a chelating agent which inhibit the degradation of the retinoid in the aqueous gel formulation. Preferred antioxidants are BHT, BHA, alpha-tocopherol and ascorbic acidin the preferred range of about 0.01% to 0.3% and more preferably BHT in the range of 0.03% to 0.1% by weight by total weight of the composition. Preferably, the chelating agent is present in an amount of from 0.01% to 0.5% by weight by total weight of the composition. Particularly preferred chelating agents include edetate salts (e.g. diso dium edetate) and citric acid in the range of about 0.01% to 0.% and more preferably in the range of 0.02% to 0.% by weight by total weight of the composition. The chelating agent is useful for chelating metal ions in the composition which may be detrimental to the shelflife of the formulation. While BHT and disodium edetate are the particularly pre ferred antioxidant and chelating agent respectively, other Suitable and equivalent antioxidants and chelating agents may be substituted therefor as would be known to those skilled in the art.
4 5 The aqueous gel composition of the invention comprises a gelling agent which provides body to the aqueous gel vehicle and maintains the dispersion of retinoids in the vehicle by maintenance of the semisolid dosage form. Par ticularly preferred gelling agents are selected from the group consisting of acrylic acid polymers cross-linked with poly alkylsucrose; and poloxamer. More particularly, the gelling agent is an acrylic acid polymer crosslinked with the allyl ether of sucrose or pentaerythritol which are generally known as carbomers. Carbomers are sold under the trade mark CARBOPOL, in various grades. The more useful grades for the purposes of the present invention are 934, 934P, 940, 941, 980 and 981 with 940 being the most preferred. These numbers designate the molecular weight and the crosslinking of the carboxypolymethylene mol ecules. Carbomers are referenced and listed in USP XXII, (1990). While the gelling agents specifically identified above are presently preferred, other suitable and equivalent gelling agents may be substituted therefor as would be known to those skilled in the art, In other optional embodiments of the invention polyvi nylpyrrollidone is added to the aqueous gel composition of the invention to inhibit crystal growth of the micronized retinoid and particularly, tretinoin. Optionally, about 0.1% to 1.0%, and more preferably, 0.3% of polyvinylpyrrolidone by weight by total weight of the composition is added to the aqueous gel. Further, other additives such as about 5% to % hydroxypropyl-beta-cyclodextrin (HPBCD) may be added to the formulation to enhance the availability of retinoid to the skin with reduced irritation. To achieve proper gelling of the formulation, the acidic carbomer gelling agent component is neutralized by the addition of a base thereto to form a gelling composition which provides body to the gel dosage form. In preferred embodiments enough base is added to the carbomer con taining aqueous gel compositions of the invention to provide a ph of between 4.0 and 7.0, more preferably 4.5 to 5.5. While any suitable base may be used the preferred bases used to raise the ph of the aqueous gels of the invention are sodium hydroxide and triethanolamine. The exact amount of sodium hydroxide or triethanolamine that is added to an aqueous gel to achieve the desired ph range depends upon the amount of carbomer that is present in the aqueous gel composition. Generally, about 0.1% to 0.6% of pure sodium hydroxide by weight by total weight of the composition is added to provide a ph of the aqueous gel composition of the invention of between 4.0 and 7.0, more preferably 4.5 to 5.5. Sodium hydroxide may be added to the aqueous gel com positions by means of a % aqueous solution for ease of handling and mixing. It is preferable in the aqueous gel composition of the invention to eliminate the presence of any water soluble alcohol such as isopropyl alcohol or ethanol from the formulation to prevent the potential deleterious affects of such alcohols, e.g. undesirable drying of the skin. While benzyl alcohol is a preferred antimicrobial preservative which is provided in amounts of about 0.5% to 2.0% by weight by total weight of the invention, it is not intended as a solubilizing alcohol as distinguished from water soluble alkyl alcohols which are intended as solubilizers for retin oids e.g. isopropanol or ethanol. The aqueous gel vehicle of the invention advantageously provides micronized retinoid as a dispersion and not a solution for topical application. The use of micronized retinoids and/or tretinoin particles represent a significant aspect of the present invention. The use of micronized particles of a water insoluble retinoid permits the use of an aqueous gel vehicle containing no solubilizing alcohol (e.g. no water soluble lower alkyl alcohol), to provide otherwise unattainable amounts of topi cal availability and penetration of retinoid into the skin. The. 6 dispersion of the "extremely fine" micronized particles achieve good penetration of the retinoid into the skin with out significant passing of retinoid through the skin. Additional medicaments or active components may be used in combination with the retinoid dosage form compo sition of the invention. For example, antibiotics used in acne preparations such as: the antibacterials erythromycin, clindamycin, tetracycline, minocycline, of loxacin and sodium sulfacetamide; antifungals such as miconazole, terconazole, ketoconazole, econazole, fluconazole and clo trimazole; corticosteriods such as triancinolone, betametha sone and clobetasol; as well as other actives such as sulfur, anthralin, azelaic acid, alpha-hydroxy acids, benzoyl perox ide and skin growth factors; salts and derivatives of all of the above; and mixtures of any of the above may be added to the retinoid dosage form of the invention. As will be discussed below in the Examples section and the evaluation thereof, the aqueous gel formulations of the invention utilizing micronized tretinoin particles dispersed informulation components provide for increased therapeutic effectiveness of tretinoin for topical application to human skin. This result is unexpected and surprising in view of the state of the prior art which is focused on solubilizing retinoids in topical formulations. The present invention surprisingly achieves excellent topical skin penetration of retinoids in an aqueous dispersion while reducing deleteri ous side effects, e.g. irritation, thus improving the therapeu tic index of the composition of the invention over those retinoid preparations of the prior art. The invention will now be illustrated by examples. The examples are not intended to be limiting of the scope of the present invention but read in conjunction with the detailed and general description above, provide further understand ing of the invention and outline a process for preparing the compositions of the inventions and methods of practicing the invention. EXAMPLES The following ingredients, processes and procedures for preparing the compositions of the present invention corre spond to that described above. The procedures below describe with particularity the various formulation ingredi ents and procedures utilized. Any methods, starting materials, reagents or excipients which are not particularly described will be generally known and available to those skilled in the pharmaceutical formulation arts. All formula tion percentages are provided in percentage by weight by total weight of the composition. Example 1 Micronized tretinoin ( %)aqueous gels with a preferred preservative system: micronized tretinoin % octoxynol-13 (IGEPAL*CA-7) 0.2% sorbic acid 0.1% benzyl alcohol 1.0% disodium edietate 0.05% ascorbic acid 0.1% sodium metabisulfite 0.2% carbomer (CARBOPOL 940) 1.5% sodium hydroxide 0.2% purified water qs 0% The aqueous gel of Example 1 was evaluated in an in vivo comparative study against a conventional alcohol gel. A formulation of a commercially available alcoholic gel in either of two strengths, 0.0% or 0.01% tretinoin, (vitamin A acid) by weight, comprises butylated hydroxytoluene,
5 7 hydroxypropyl cellulose and alcohol in a gel vehicle. This formulation is utilized throughout the Examples section and designated as "". An evaluation and comparative of the aqueous gel of the invention and this conventional alcohol gel is provided below. The tests were carried out in accordance with the protocol and systems provided and explained in U.S. Pat. No. 4487,782 and Mezicket al., Topical and Systemic Effects of Retinoids on Horn-Filled Utriculus Size in the Rhino Mouse. AModel to Quantify 'Antikeratinizing Effects of Retinoids,Journal of Investigative Dermatology, 83:1-113(1984), the entire disclosure of which is hereby incorporated herein by refer CCC. In the following formulation evaluation EDso refers to measurements of efficacy (% utriculus reduction); IDso refers to irritation (% of maximum erythema grade of 3.0); Rel. Potency is relative potency and F.L. is the fiducial limit. Rhino Mouse Utriculus Reduction %. Utriculus Rel. Potency Dose a Reduction EDs (95% F.L.) O.O Aqueous Gel (Example 1) 0, (1.2, 2.6) Rabbit Dermal titation Erythema Rel. Potency Dose % Grade IDso (95% F.L.) OOO OS Aqueous Gel (Example 1) (0.05, 0.16) The aqueous gel of Example 1 is found to be more potent than the comparative alcohol gel on utriculus reduction, as demonstrated by the lower EDso for the aqueous gel and much less irritating to rabbit skin as indicated by the higher tolerated dose at IDso for the aqueous gel. The therapeutic index comprising the ratio of IDs/EDs (TL) for the aqueous gel of the invention is 0.1/0.006 (TI-17) vs. the comparative alcohol gel which is 0.007/0.008 (T.I.=1). Example 2 tretinoin 0.0% octoxynol-13 (IGEPALCA-7) 0.2% sorbic acid 0.1% benzyl alcohol 1.0% disodium edietate 0.05% alpha-tocopherol 0.04% carbomer (CARBOPOL 940) 1.5% sodium hydroxide 0.2% purified water qs 0% This formulation was tested for the extent of tretinoin penetration into human skin in vitro. The following proce 5 8 dure is used for investigating the in vitro skin permeation of tretinoin from the aqueous and alcoholic gel formulations: Human skin samples were kept frozen at -70 C. until use. Samples were thawed at room temperature for minutes, and then rinsed with normal saline. Skin samples were cut into appropriate sized pieces, tared, a 1 mm thick film of formulation was applied, and the samples were weighed. The skin was then mounted on a diffusion cell (modified Franz diffusion cells, 9 mm opening, ml volume), which had previously been filled with receptor medium (% HPBCD in water). Upon completion of the study (24 hours), the receptor medium was sampled. The excess formulation was wiped from the skin surface using a Kim-Wipe soaked in acetone, the skin surface was washed twice with water and once with acetone, wiping the skin surface with a Kim-Wipe after each wash. Each skin sample was placed into a volumetric flask and tretinoin was extracted. The skin samples were extracted with methanol:ethyl acetate (1:1) with 0.5% BHT. Samples were sonicated minutes and a portion of the extracts were then assayed by HPLC. The results of the in vitro skin permeation of tretinoin from the formulation of Example 2 as compared to alcoholic gel (comparative) and two other comparative formulations whereby the formulation of Example 2 was altered to exclude the surfactant and to substitute unmicronized par ticles of tretinoin for micronized particles are all summa rized below: Formulation Alcoholic Gel (comparative) Aqueous Gel (Ex. 2) Aqueous Gel (Ex. 2) without surfactant Aqueous Gel (Ex. 2) with large (unmicronized) tretinoin particles Amount in Skin 18 +/- 2 mcg +/- 1 mcg 9.3 +/- 2 mcg Amount Passing Through Skin 0.4 mcg 0.0 mcg (below detection limit) 0.0 mcg 18 +/- 2 mcg 0.0 mcg The tretinoin aqueous gel of the presentinvention releases tretinoin into the upper layer of human skin but not any detectable amount passes through the skin. This example of an aqueous gel of the invention thus releases tretinoin into the skin as well as or better than the commercially available alcoholic gels without excess drug passing through the skin and without excess irritation. Further investigation of the aqueous gels of the invention versus comparison gels not containing a surfactant or con taining larger (unmicronized) particles of tretinoin indicates a drop in skin permeation of tretinoin of approximately 3-fold and 2-fold respectively. Such results indicate that the use of a surfactant and micronized particles of tretinoin contribute to a surprising enhancement of tretinoin skin permeation from the aqueous gel formulations of the invention.
6 9 Example 3 Formulation Evaluation Micronized tretinoin ( %) aqueous gels with polyvinylpyrrolidone: micronized tretinoin % octoxynol-13 (IGEPAL*CA-7) 0.2% methyl paraben 0.18% propyl paraben 0.02% benzyl alcohol 1.0% disodium edietate 0.05% ascorbic acid 0.1% polyvinylpyrrollidone 0.3% carbomer (CARBOPOL*934) 1.5% sodium hydroxide 0.16% purified water qs 0% Formulation Evaluation Rhino Mouse Utriculus Reduction %. Utriculus Rel. Potency Dose % Reduction EDso (95% FL.) Aqueous Gel (Example 3) (0.7, 1.1) Rabbit Dermal Erritation Erythema Rel. Potency Dose % Grade Dso (95% FL.) Aqueous Gel (Example 3) (0.1, 07) This aqueous gel is found to be as potent as the alcohol gel on utriculus reduction, but was much less irritating to rabbit skin (aqueous gel T.L= vs. alcohol gel T.I.=2.5). Example 4 micronized tretinoin % octoxynol-13 (IGEPAL* CA-7) 0.2% disodium edietate 0.02% ascorbic acid 0.1% imidurea (GERMALL* 1) 0.4% polyvinylpyrrolidone 0.3% hydroxypropyl-beta-cyclodextrin.0% carbomer (CARBOPOL*940) 1.5% sodium hydroxide 0.18% purified water qs 0% Rhino Mouse Utriculus Reduction % Utriculus Rel. Potency Dose % Reduction EDso (95% F.L.) Aqueous Gel (Example 4) (0.6, 1.4) Rabbit Dermal Irritation Erythema Rel. Potency Dose % Grade IDso (95% F.L.) Aqueous Gel (Example 4) O (0.08, 0.5) The above aqueous gel is found to be as potent as the alcohol gel on utriculus reduction, but was much less irritating to rabbit skin (aqueous gel TL=24 vs. alcohol gel TL=2.5). Example 5 micronized tretinoin % octoxynol-13 (IGEPAL* CAT) 0.2% sorbic acid, N.F 0.1% benzyl alcohol, NF 1.0% disodium edetate, NF 0.05% citric acid, USP 0.1% butylated hydroxytoluene, NF 0.05% carbomer (CARBOPOL*940), NF 1.5% sodium hydroxide, USP 0.2% purified water, USP qs 0% The above tretinoin aqueous gel composition is expected to provide similar advantageous results analogous to those formulations of the invention evaluated above. The above evaluations and test results demonstrate the utility of the formulations of the invention for increasing the effective therapeutic index, i.e. Do/EDso for topical admin istration of retinoids, particularly, tretinoin on the skin of a patient. The increased therapeutic index is achieved by requiring higher amounts of active (retinoid) to produce a specific threshold of irritation (IDs) and lower amounts to produce a specific threshold of efficacy (EDs) in the aque ous gel formulations of the invention. The scope of the present invention is not limited by the description, examples and suggested uses herein and modi fications can be made without departing from the spirit of the invention. The formulations of the invention may, for example, have other applications and uses in addition to those described herein. Applications of the compositions and methods of the present invention for medical or pharmaceutical uses can be
7 11 accomplished by any clinical, medical, and pharmaceutical methods and techniques as are presently or prospectively known to those skilled in the art. Thus it is intended that the invention cover any modifications and variations of this invention provided that they come within the scope of the appended claims and their equivalents. What is claimed is: 1. A tretinoin aqueous gel dispersion composition for therapeutic topical administration of tretinoin to the skin comprising: a therapeutically effective amount of unsolubi lized micronized tretinoin particles; a surfactant selected from the group consisting of octoxynol and nonoxynol in an amount effective to enhance penetration of tretinoin into the skin; a preservative; a gelling agent in an amount Sufficient to provide body to the aqueous gel dosage form skin and which maintains the dispersion of tretinoin in the composi tion by maintenance of a semisolid dosage form; and water qs to 0%. 2. A tretinoin aqueous gel dispersion composition for topical administration of tretinoin to the skin comprising in weight by total weight of the composition: to 0.5% micronized tretinoin particles; to 1.0% surfactant selected from the group consisting of octoxynol and non oxynol; to 2.0% preservative; 0.01% to 0.3% antioxi dant; 1.0% to 2.0% of a gelling agent; sufficient base to attain a ph in the range of from 4.0 to 7.0; and water qs to 0%. 3. An aqueous gel composition according to claim 1 wherein the preservative is selected from the group consist ing of: benzyl alcohol, sorbic acid, parabens, imidiurea and combinations thereof. 4. An aqueous gel composition according to claim 1 wherein the gelling agent is selected from the group con sisting of: acrylic acid polymer crosslinked with allyl ether of sucrose or pentaerythritol; and poloxamer. 5. An aqueous gel composition according to claim 1 wherein the micronized tretinoin comprises at least 90% of the particles in the size range of 1 to microns. 6. An aqueous gel composition according to claim 1 wherein the micronized tretinoin particles have a mean size in the range of 1 to microns. 7. An aqueous gel composition according to claim 1 wherein polyvinylpyrrolidone is added in an amount effec tive to inhibit crystal growth of the micronized tretinoin. 8. An aqueous gel composition according to claim 2 wherein the micronized tretinoin is present in the range of to 0.2%. 9. An aqueous gel composition according to claim 2 wherein the surfactant is present in the range of 0.01 to 0.5%.. An aqueous gel composition according to claim 1 additionally comprising a chelating agent. 11. An aqueous gel composition according to claim 1 additionally comprising hydroxypropyl-beta-cyclodextrin. 12. An aqueous gel composition according to claim 2 wherein the base used to lower the ph of the composition to a ph between 4.0 to 7.0 is sodium hydroxide or triethano lamine. 13. An aqueous gel composition according to claim wherein about 0.1 to 0.6% sodium hydroxide is added to provide a ph to the composition of between 4.5 to An aqueous gel composition according to claim 2 wherein the antioxidantis selected from the group consisting of: alpha-tocopherol, butylated hydroxytoluene, butylated hydroxyanisole and ascorbic acid.. An aqueous gel composition according to claim 1 which does not contain any water soluble lower alkyl alcohol A method of increasing the therapeutic effectiveness of tretinoin for topical application to the skin comprising the step of delivering micronized tretinoin dispersed in an aqueous gel vehicle containing a surfactant selected from the group consisting of octoxynol and nonoxynol to the intended site of skin application. 17. A method of increasing the therapeutic effectiveness of tretinoin for topical skin application comprising the step of delivering micronized tretinoin to the intended site of application using an aqueous gel dispersion composition in accordance with claim The method of claim 17 wherein the aqueous gel composition contains no water soluble lower alkyl alcohol. 19. A method of improving the skin penetration of a topically administered tretinoin to the skin of a patient comprising the steps of: dispersing micronized tretinoin in an aqueous gel com position in accordance with claim 1; and applying the tretinoin aqueous gel to the skin of a patient.. A method of reducing irritation associated with the topical administration of tretinoin to a patient comprising the steps of: dispersing micronized tretinoin in an aqueous gel com position in accordance with claim 1; and applying the micronized tretinoin aqueous gel to the skin of a patient. 21. A retinoid aqueous gel composition for therapeutic topical administration of retinoid to the skin comprising: a therapeutically effective amount of unsolubilized micron ized retinoid particles; a surfactant selected from the group consisting of octoxynol and nonoxynol in an amount effec tive to enhance penetration of retinoid into the skin; a preservative; a gelling agent composition in an amount sufficient to provide body to the aqueous gel dosage form and which maintains the dispersion of retinoids in the composition by maintenance of a semisolid dosage form; and water qs to 0%. 22. A retinoid aqueous gel dispersion composition for topical administration of retinoid to the skin comprising in weight by total weight of the composition: to 0.5% micronized unsolubilized retinoid particles; to 1.0% surfactant selected from the group consisting of octoxynol and nonoxynol; to 2.0% preservative; 0.01% to 0.3% antioxidant; 1.0% to 2.0% of a gelling agent; sufficient base to attain a ph in the range of from 4.0 to 7.0; and water qs to 0%. 23. An aqueous gel composition according to claim 21 wherein the micronized retinoid comprises at least 90% of particles in the range of from in 1 to microns. 24. An aqueous gel composition according to claim 21 wherein the micronized retinoid particles have a mean size in the range of 1 to microns.. An aqueous gel composition according to claim 21 additionally comprising a chelating agent. 26. An aqueous gel composition according to claim 22 wherein the chelating agent is selected from the group consisting of disodium edetate and citric acid. 27. A method of increasing the therapeutic effectiveness of retinoid for topical application to the skin comprising the step of delivering micronized retinoid dispersed in an aque ous gel vehicle containing a surfactant selected from the group consisting of octoxynol and nonoxynol to the intended site of skin application. 28. The method of claim 27 wherein the aqueous gel vehicle contains no water soluble lower alkyl alcohol. 29. A tretinoin aqueous gel dispersion composition for therapeutic topical administration of tretinoin to the skin
8 13 consisting essentially of in weight by total weight of the composition: to 0.5% micronized unsolubilized tret inoin particles; to 1.0% surfactant selected from the group consisting of octoxynol and monoxynol; to 2.0% preservative; 0.01% to 0.3% antioxidant; 1.0% to 2.0% of a gelling agent; sufficient base to attain a ph in the range of from 4.0 to 7.0; and water.. A retinoid aqueous gel dispersion composition for therapeutic topical administration of retinoid to the skin consisting essentially of a therapeutically effective amount of unsolublized micronized retinoid particles; a surfactant selected From the group consisting of octoxynol and non Oxynol in an amount effective to enhance penetration of retinoid into the skin; a preservative; a gelling agent com position in an amount sufficient to provide body to the aqueous gel dosage form; and Water. 31. A method for reducing irritation associated with the topical administration of tretinoin to the skin of a patient comprising: (a) dispersing in a composition for topical administration in weight by total weight of the composition: to 0.5% micronized unsolubilized tretinoin particles; to 1.0% surfactant selected from the group con sisting of octoxynol and nonoxynol; to 2.0% preservative; 0.01% to 0.3% antioxidant; 1.0% to 2.0% of a gelling agent; sufficient base to attain a ph in the range of from 4.0 to 7.0; and water; and (b) applying said composition to the skin of a patient. 5 O A method for reducing irritation associated with the topical administration of a retinoid to the skin of a patient comprising: (a) dispersing in a composition for topical administration in weight by total weight of the composition: to 0.5% micronized unsolubilized retinoid particles; to 1.0% surfactant selected from the group consisting of octoxynol and nonoxynol; to 2.0% preserva tive; 0.01% to 0.3% antioxidant; 1.0% to 2.0% of a gelling agent; sufficient base to attain a ph in the range of from 4.0 to 7.0; and water; and (b) applying said composition to the skin of a patient. 33. A method for reducing irritation associated with the topical administration of a retinoid to the skin of a patient while maintaining therapeutic effectiveness of said retinoid comprising: (a) dispersing in a composition for topical administration comprising: a therapeutically effective mount of micronized retinoid particles; a surfactant selected from the group consisting of octoxynol and nonoxynol in an amount effective to enhance penetration of retin oid into the skin; a preservative; a gelling agent com position in an amount sufficient to provide body to the aqueous gel dosage form; and water qs to 0%; and (b) applying said composition to the skin era patient. ; :: :: * ::
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0165220 A1 Chang et al. US 201701 65220A1 (43) Pub. Date: (54) (71) (72) (21) (22) (60) (60) TOPCAL PHARMACEUTICAL FORMULATIONS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0165220 A1 Chang et al. US 201701 65220A1 (43) Pub. Date: (54) (71) (72) (21) (22) (60) (60) TOPCAL PHARMACEUTICAL FORMULATIONS
(12) United States Patent (10) Patent No.: US 6,841,523 B1
 USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
III. United States Patent 19 Jordan 5,389,129. Feb. 14, ). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee:
 United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O130248A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0130248A1 MOZZOne et al. (43) Pub. Date: (54) TOPICAL ANTI-INFLAMMATORY COMPOSITIONS (76) Inventors: Keith
(19) United States US 2003O130248A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0130248A1 MOZZOne et al. (43) Pub. Date: (54) TOPICAL ANTI-INFLAMMATORY COMPOSITIONS (76) Inventors: Keith
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
United States Patent (19) Katz
 United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
(12) United States Patent (10) Patent No.: US 6,752,627 B2
 USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
Evaluation of Cosmeceutical Ingredients: What the Label May Not Reveal Patrick Bitter, MD. Regulation of Topical Skin Care Products.
 Evaluation of Cosmeceutical Ingredients: What the Label May Not Reveal Patrick Bitter, MD Regulation of Topical Skin Care Products US Food and Drug Administration (FDA) recognizes two categories of products
Evaluation of Cosmeceutical Ingredients: What the Label May Not Reveal Patrick Bitter, MD Regulation of Topical Skin Care Products US Food and Drug Administration (FDA) recognizes two categories of products
Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents
 Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents Sharing expertise around the world As a global manufacturer of cosmetic ingredients and functional fine chemicals, Hannong Chemicals
Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents Sharing expertise around the world As a global manufacturer of cosmetic ingredients and functional fine chemicals, Hannong Chemicals
12) United States Patent 10) Patent No.: US 6,433,024 B1
 USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
(12) United States Patent
 US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
CREAMS BATH SOAP SUBSTITUTES OINTMENT GEL ADDITIVES. NEW 500g. pump
 CREAMS BATH ADDITIVES SOAP SUBSTITUTES OINTMENT GEL NEW NEW 500g pump The Zeroderma range of emollients offers similar products to leading brands with no compromise on patient care and cost s of up to
CREAMS BATH ADDITIVES SOAP SUBSTITUTES OINTMENT GEL NEW NEW 500g pump The Zeroderma range of emollients offers similar products to leading brands with no compromise on patient care and cost s of up to
TAGRAVIT TM R1 Encapsulated pure retinol. March 2015
 TAGRAVIT TM R1 Encapsulated pure retinol March 2015 Retinol- Background Retinol is a form of Vitamin A having a small molecular structure with MW of 286.45 g/mol. Retinol can penetrate the outer layers
TAGRAVIT TM R1 Encapsulated pure retinol March 2015 Retinol- Background Retinol is a form of Vitamin A having a small molecular structure with MW of 286.45 g/mol. Retinol can penetrate the outer layers
Design, development and evaluation of solid dispersion incorporated transdermal gel of benzoyl peroxide
 2016; 5(7): 13-18 ISSN: 2277-7695 TPI 2016; 5(7): 13-18 2016 TPI www.thepharmajournal.com Received: 01-05-2016 Accepted: 02-06-2016 Jitender Mor Rajinder Mann Bharti Chopra Design, development and evaluation
2016; 5(7): 13-18 ISSN: 2277-7695 TPI 2016; 5(7): 13-18 2016 TPI www.thepharmajournal.com Received: 01-05-2016 Accepted: 02-06-2016 Jitender Mor Rajinder Mann Bharti Chopra Design, development and evaluation
United States Patent (19)
 United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
United States Patent (19) Humbrecht
 United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(12) United States Patent (10) Patent No.: US 6,308,717 B1
 USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
Europaisches Patentamt European Patent Office. Publication number: Office europeen des brevets EUROPEAN PATENT APPLICATION
 J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
Irwin Palefsky Cosmetech Laboratories Inc.
 Irwin Palefsky Cosmetech Laboratories Inc. Irwin@cosmetech.com www.cosmetech.com The prevention or retardation of product deterioration caused by microorganisms from the time of production until the product
Irwin Palefsky Cosmetech Laboratories Inc. Irwin@cosmetech.com www.cosmetech.com The prevention or retardation of product deterioration caused by microorganisms from the time of production until the product
The solution contains isopropyl alcohol 50% v/v, propylene glycol, and water.
 Cleocin T (clindamycin phosphate topical solution, USP) (clindamycin phosphate topical gel) (clindamycin phosphate topical lotion) For External Use DESCRIPTION CLEOCIN T Topical Solution and CLEOCIN T
Cleocin T (clindamycin phosphate topical solution, USP) (clindamycin phosphate topical gel) (clindamycin phosphate topical lotion) For External Use DESCRIPTION CLEOCIN T Topical Solution and CLEOCIN T
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
(19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
The Effects of Shear on Neutralized Carbomers in Aqueous Conditions
 The Effects of Shear on Neutralized Carbomers in Aqueous Conditions Lyndel Speedy 18/07/2014 With thanks to Ensign Laboratories 1 Abstract Carbomer is the generic name for a class of high molecular weight
The Effects of Shear on Neutralized Carbomers in Aqueous Conditions Lyndel Speedy 18/07/2014 With thanks to Ensign Laboratories 1 Abstract Carbomer is the generic name for a class of high molecular weight
(12) United States Patent (10) Patent No.: US 6,413,305 B1
 USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes
 Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes Chapter 18 (Viscosity-inducing Agents) 1. What is Carbomer NF? 2. Carbomer is commonly referred to
Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes Chapter 18 (Viscosity-inducing Agents) 1. What is Carbomer NF? 2. Carbomer is commonly referred to
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150343112A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0343112 A1 Dyar et al. (43) Pub. Date: (54) LIQUID BANDAGE Publication Classification (71) Applicant: PROBLEM
(19) United States US 20150343112A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0343112 A1 Dyar et al. (43) Pub. Date: (54) LIQUID BANDAGE Publication Classification (71) Applicant: PROBLEM
United States Patent (19)
 United States Patent (19) Young 54) THERAPEUTIC COMPOSITION (75) Inventor: Henry Y. Young, Delmar, N.Y. 73) Assignee: Stiefel Laboratories, Inc., Oak Hill, N.Y. (21) Appl. No.: 1,773. 22). Filed: Apr.
United States Patent (19) Young 54) THERAPEUTIC COMPOSITION (75) Inventor: Henry Y. Young, Delmar, N.Y. 73) Assignee: Stiefel Laboratories, Inc., Oak Hill, N.Y. (21) Appl. No.: 1,773. 22). Filed: Apr.
Ref. 11. (12) Patent Application Publication (10) Pub. No.: US 2012/ A1. (19) United States. Polstein et al. (43) Pub. Date: Jun.
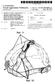 (19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(12) United States Patent
 US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
PRODUCT INFORMATION DIFFERIN ADAPALENE 0.1% TOPICAL GEL
 PRODUCT INFORMATION DIFFERIN ADAPALENE 0.1% TOPICAL GEL NAME OF THE MEDICINE DIFFERIN Topical Gel: Adapalene 1 mg/g (0.1%) Common Name: Adapalene Chemical Name: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic
PRODUCT INFORMATION DIFFERIN ADAPALENE 0.1% TOPICAL GEL NAME OF THE MEDICINE DIFFERIN Topical Gel: Adapalene 1 mg/g (0.1%) Common Name: Adapalene Chemical Name: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic
United States Patent (19)
 USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
(*) Notice: Slity sight 58 $5 E. G.
 USOO629.7208B1 (12) United States Patent (10) Patent No.: US 6,297,208 B1 Crist () Date of Patent: Oct. 2, 2001 (54) RUST STAIN REMOVAL FORMULA 4,891,0 1/1990 Gross et al.. 4,963,233 * 10/1990 Mathew...
USOO629.7208B1 (12) United States Patent (10) Patent No.: US 6,297,208 B1 Crist () Date of Patent: Oct. 2, 2001 (54) RUST STAIN REMOVAL FORMULA 4,891,0 1/1990 Gross et al.. 4,963,233 * 10/1990 Mathew...
United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985
 United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985 54 INK RIBBON CASSETTE PROVIDED WITH 56) References Cited AN EMPREGNATION DEVICE U.S. PATENT DOCUMENTS s 2,76,539
United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985 54 INK RIBBON CASSETTE PROVIDED WITH 56) References Cited AN EMPREGNATION DEVICE U.S. PATENT DOCUMENTS s 2,76,539
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
(19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
THE ROLE OF BIO-IDENTICAL HORMONES IN HEALTH AND BEAUTY. Copyright 2011 Wholistic Dermatology
 THE ROLE OF BIO-IDENTICAL HORMONES IN HEALTH AND BEAUTY Copyright 2011 Wholistic Dermatology WHY WHOLISTIC DERMATOLOGY VS. TRADITIONAL METHODOLOGY Wholistic means addressing and treating the body as a
THE ROLE OF BIO-IDENTICAL HORMONES IN HEALTH AND BEAUTY Copyright 2011 Wholistic Dermatology WHY WHOLISTIC DERMATOLOGY VS. TRADITIONAL METHODOLOGY Wholistic means addressing and treating the body as a
DAKTARIN. Miconazole, Miconazole nitrate CREAM, POWDER, SPRAY POWDER, LOTION, TINCTURE PRODUCT INFORMATION
 DAKTARIN Miconazole, Miconazole nitrate CREAM, POWDER, SPRAY POWDER, LOTION, TINCTURE PRODUCT INFORMATION Description Miconazole is 1-[2,4-dichloro-beta-(2,4-dichlorobenzyloxy)phenethyl]imidazole derivative
DAKTARIN Miconazole, Miconazole nitrate CREAM, POWDER, SPRAY POWDER, LOTION, TINCTURE PRODUCT INFORMATION Description Miconazole is 1-[2,4-dichloro-beta-(2,4-dichlorobenzyloxy)phenethyl]imidazole derivative
Item/Package Details Size Item Bottle ph Shelf Life 1.0 oz/29.6 ml 1101 Lucite Matte Silver Pump months
 B-Kleer 10 Lotion Use of mild to moderate acne. Gently exfoliates encrustation and acne pustules. Penetrates pores to eliminate most acne pimples. Reduces the severity of acne blemishes. Help prevent new
B-Kleer 10 Lotion Use of mild to moderate acne. Gently exfoliates encrustation and acne pustules. Penetrates pores to eliminate most acne pimples. Reduces the severity of acne blemishes. Help prevent new
Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries.
 Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. I. Preservation Naturally-derived product Broad spectrum protection Globally accepted II. Moisturization
Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. I. Preservation Naturally-derived product Broad spectrum protection Globally accepted II. Moisturization
There are, however, long-term effects of UV radiation, which are irreversible and often malignant.
 Sun Care Products Skin exposure affects the skin in many ways. In the short term, it can lead to reddening, irritation, and eventually tanning, which is the main reason for most people sunbathing. There
Sun Care Products Skin exposure affects the skin in many ways. In the short term, it can lead to reddening, irritation, and eventually tanning, which is the main reason for most people sunbathing. There
Unisooth EG-28 Rapid Control of Skin Irritation for the removal of Dark Circles
 Unisooth EG-28 Rapid Control of Skin Irritation for the removal of Dark Circles The appearance of dark circles is a biologically complex process closely linked to subocular micro-inflammation. By actively
Unisooth EG-28 Rapid Control of Skin Irritation for the removal of Dark Circles The appearance of dark circles is a biologically complex process closely linked to subocular micro-inflammation. By actively
Int. Cl."... F21V1/06 U.S. C /352; 362/358. References Cited U.S. PATENT DOCUMENTS 3,787,676 l/1974 Korach /352
 United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
PO Box 5411 Arlington, TX SF A-348
 SF A-348 PRODUCT DESCRIPTION SF A-348 organosilicone is a proprietary copolymer that represents a class of aminosilicone polyalkyleneoxide copolymers for hair conditioning. Conventional organomodified
SF A-348 PRODUCT DESCRIPTION SF A-348 organosilicone is a proprietary copolymer that represents a class of aminosilicone polyalkyleneoxide copolymers for hair conditioning. Conventional organomodified
Performance is in our nature.
 Performance is in our nature. 2 TECHNICAL BULLETIN USP-FCC Propanediol Making Beverages Better, Cheaper and Faster Abstract: USP-FCC propanediol is a bio-based polyol that adds a sweet, cool taste to beverages,
Performance is in our nature. 2 TECHNICAL BULLETIN USP-FCC Propanediol Making Beverages Better, Cheaper and Faster Abstract: USP-FCC propanediol is a bio-based polyol that adds a sweet, cool taste to beverages,
(12) United States Patent
 (12) United States Patent USOO7374282B2 (10) Patent No.: US 7,374.282 B2 Tendler (45) Date of Patent: May 20, 2008 (54) METHOD AND APPARATUS FOR VIEWING 6,623,116 B2 * 9/2003 Kerns et al.... 351,165 POLARIZED
(12) United States Patent USOO7374282B2 (10) Patent No.: US 7,374.282 B2 Tendler (45) Date of Patent: May 20, 2008 (54) METHOD AND APPARATUS FOR VIEWING 6,623,116 B2 * 9/2003 Kerns et al.... 351,165 POLARIZED
COSMETIC INTENSIVE TREATMENT (CIT) INSTRUMENTS
 COSMETIC INTENSIVE TREATMENT (CIT) INSTRUMENTS DESCRIPTION: Roll-CIT : A hand-held rolling instrument with a smooth action roller head embedded with durable stainless steel micro-needles that protrude
COSMETIC INTENSIVE TREATMENT (CIT) INSTRUMENTS DESCRIPTION: Roll-CIT : A hand-held rolling instrument with a smooth action roller head embedded with durable stainless steel micro-needles that protrude
(12) United States Patent
 (12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
(12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
United States Patent (19) 11 4,344,932 Gordon 45 Aug. 17, 1982
 United States Patent (19) 11 Gordon 45 Aug. 17, 1982 54 NAIL CLEANSER 2,764,168 9/1956 Herz... 132/73 3,1,048 9/1964 Hollab... 424/6 (75) Inventor: Harry W. Gordon, New York, N.Y. 3,384,592 5/1968 Weems...
United States Patent (19) 11 Gordon 45 Aug. 17, 1982 54 NAIL CLEANSER 2,764,168 9/1956 Herz... 132/73 3,1,048 9/1964 Hollab... 424/6 (75) Inventor: Harry W. Gordon, New York, N.Y. 3,384,592 5/1968 Weems...
BARNET CORNEOTHERAPY RESURFACID CR. AHA s Normalization of Increased Skin s ph Time Release Technology Ultra Mild Exfoliation
 BARNET CORNEOTHERAPY RESURFACID CR AHA s Normalization of Increased Skin s ph Time Release Technology Ultra Mild Exfoliation The information contained in this technical bulletin is, to the best of our
BARNET CORNEOTHERAPY RESURFACID CR AHA s Normalization of Increased Skin s ph Time Release Technology Ultra Mild Exfoliation The information contained in this technical bulletin is, to the best of our
Identification and quantification of preservative chemicals in common household products. Session 1
 Background Session 1 Preservatives are chemicals that are commonly added to food or general such as toiletries and pharmaceuticals in order to increase their shelf lives. Preservatives can act as antimicrobials,
Background Session 1 Preservatives are chemicals that are commonly added to food or general such as toiletries and pharmaceuticals in order to increase their shelf lives. Preservatives can act as antimicrobials,
United States Patent (19)
 United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
SunCat MTA. Safe and Efficient Sunscreen Dispersion
 SunCat MTA Safe and Efficient Sunscreen Dispersion % Reaching Ground 95% 5% UVA UVB UVC Causes Aging Sunscreen Protection Causes Burning & Tanning Blocked by Atmosphere 12 STAR SYSTEM SUN PROTECTION FACTOR
SunCat MTA Safe and Efficient Sunscreen Dispersion % Reaching Ground 95% 5% UVA UVB UVC Causes Aging Sunscreen Protection Causes Burning & Tanning Blocked by Atmosphere 12 STAR SYSTEM SUN PROTECTION FACTOR
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(12) United States Patent (10) Patent No.: US 7434,929 B2
 US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O18O194A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180194 A1 Cutter (43) Pub. Date: Jul.19, 2012 (54) GARMENTS WITH ADJUSTABLE WAISTS (52) U.S. Cl.... 2/237
(19) United States US 2012O18O194A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180194 A1 Cutter (43) Pub. Date: Jul.19, 2012 (54) GARMENTS WITH ADJUSTABLE WAISTS (52) U.S. Cl.... 2/237
Nov. 18, 1969 J. B. MARTN, JR 3,478,754 APPLICATOR FOR FALSE EYELASHES
 Nov. 18, 1969 J. B. MARTN, JR 3,478,754 Filed April 30, 1968 3 Sheets-Sheet Nov. 18, 1969 J, B, MARTIN, JR 3,478,754 3 sheets-sheet 2 Filed April 30, 1968 INVENTOR. W womes A. Marr'r, V. Nov. 18, 1969
Nov. 18, 1969 J. B. MARTN, JR 3,478,754 Filed April 30, 1968 3 Sheets-Sheet Nov. 18, 1969 J, B, MARTIN, JR 3,478,754 3 sheets-sheet 2 Filed April 30, 1968 INVENTOR. W womes A. Marr'r, V. Nov. 18, 1969
LaraCare A200 Your Multi-Functional Larch Tree Active
 PersonalCare LaraCare A200 Your Multi-Functional Larch Tree Active INCI Name: Galactoarabinan SAP #: 179796 Key Product Attributes: Reduce Transepidermal Water Loss Reduce appearance of fine lines and
PersonalCare LaraCare A200 Your Multi-Functional Larch Tree Active INCI Name: Galactoarabinan SAP #: 179796 Key Product Attributes: Reduce Transepidermal Water Loss Reduce appearance of fine lines and
Technology. HydroSal. Formulated for suspension in. water and hydro-alcoholic. environments, HydroSal. provides long lasting effects and
 Technology Technology specialized for the cosmeceutical and pharmaceutical industries. Solutions for the household, industrial, food and beverage industries. Partner with us to create custom encapsulations
Technology Technology specialized for the cosmeceutical and pharmaceutical industries. Solutions for the household, industrial, food and beverage industries. Partner with us to create custom encapsulations
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1. Reynolds et al. (43) Pub. Date: Jan. 15, 2004
 (19) United States US 2004001 0311A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0010311 A1 Reynolds et al. (43) Pub. Date: (54) CUSTOMIZABLE INTEGRATED (21) Appl. No.: 10/196,311 PROSTHETIC
(19) United States US 2004001 0311A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0010311 A1 Reynolds et al. (43) Pub. Date: (54) CUSTOMIZABLE INTEGRATED (21) Appl. No.: 10/196,311 PROSTHETIC
PRODUCT INFORMATION DIFFERIN Adapalene 1mg/g TOPICAL CREAM
 PRODUCT INFORMATION DIFFERIN Adapalene 1mg/g TOPICAL CREAM NAME OF THE MEDICINE Common Name: Adapalene Chemical Name: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid Molecular Formula: C 28 H 28 O
PRODUCT INFORMATION DIFFERIN Adapalene 1mg/g TOPICAL CREAM NAME OF THE MEDICINE Common Name: Adapalene Chemical Name: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid Molecular Formula: C 28 H 28 O
The secondary objective was to evaluate the cosmetic properties and its efficacy after 28 days.
 P0090 CLINICAL EVALUATION OF CUTANEOUS SAFETY AND EFFICACY OF A FACE EMULSION CONTAINING MYRTACIN EXTRACT, PP VITAMIN ANS SABAL EXTRACT ON ACNE PRONE SKIN SUBJECTS TREATED BY TOPICAL ANTI ACNE DRUG THERAPY.
P0090 CLINICAL EVALUATION OF CUTANEOUS SAFETY AND EFFICACY OF A FACE EMULSION CONTAINING MYRTACIN EXTRACT, PP VITAMIN ANS SABAL EXTRACT ON ACNE PRONE SKIN SUBJECTS TREATED BY TOPICAL ANTI ACNE DRUG THERAPY.
United States Patent 19
 United States Patent 19 Van Scott et al. (54) DITHRANOL COMPOSITIONS STABILIZED WITH ALPHA HYDROXYACDS Eugene J. Van Scott, 1138 Sewell La., Rydal, Pa. 19046; Ruey J. Yu, 4 76 Inventors: Lindenwold Ave.,
United States Patent 19 Van Scott et al. (54) DITHRANOL COMPOSITIONS STABILIZED WITH ALPHA HYDROXYACDS Eugene J. Van Scott, 1138 Sewell La., Rydal, Pa. 19046; Ruey J. Yu, 4 76 Inventors: Lindenwold Ave.,
SEBOREGULATING. Pureskin.
 SEBOREGULATING Pureskin www.provitalgroup.com Pureskin INTRODUCTION Sulphur has been used as a therapeutic agent to treat dermatological conditions from times immemorial. Its keratolytic action is due
SEBOREGULATING Pureskin www.provitalgroup.com Pureskin INTRODUCTION Sulphur has been used as a therapeutic agent to treat dermatological conditions from times immemorial. Its keratolytic action is due
Essential Elements of Rheology Control
 Essential Elements of Rheology Control Richard Giles AkzoNobel Technical Service and Development Manager Europe, Middle East, Africa and India In-cosmetics Barcelona 2015 Outline Today s trends BALANCE
Essential Elements of Rheology Control Richard Giles AkzoNobel Technical Service and Development Manager Europe, Middle East, Africa and India In-cosmetics Barcelona 2015 Outline Today s trends BALANCE
(12) United States Patent
 USOO8663699B2 (12) United States Patent Chang et al. (54) TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING ALOW CONCENTRATION OF BENZOYL PEROXDE IN SUSPENSION IN WATER ANDA WATER-MSCIBLE ORGANIC SOLVENT
USOO8663699B2 (12) United States Patent Chang et al. (54) TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING ALOW CONCENTRATION OF BENZOYL PEROXDE IN SUSPENSION IN WATER ANDA WATER-MSCIBLE ORGANIC SOLVENT
Management of acne requires proper application
 DRUG THERAPY TOPICS A Qualitative and Quantitative Assessment of the Application and Use of Topical Acne Medication by Patients James Q. Del Rosso, DO Management of acne requires proper application of
DRUG THERAPY TOPICS A Qualitative and Quantitative Assessment of the Application and Use of Topical Acne Medication by Patients James Q. Del Rosso, DO Management of acne requires proper application of
New Zealand Datasheet
 New Zealand Datasheet 1 PRODUCT NAME Epiduo 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% / benzoyl peroxide 2.5% gel 3 PHARMACEUTICAL FORM Epiduo is a white to very pale yellow opaque gel
New Zealand Datasheet 1 PRODUCT NAME Epiduo 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% / benzoyl peroxide 2.5% gel 3 PHARMACEUTICAL FORM Epiduo is a white to very pale yellow opaque gel
Children s Hospital Of Wisconsin
 Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
PDF of Trial CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Tue, 02 Oct 2018 21:40:33 GMT) CTRI Number Last Modified On 26/12/2012 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Tue, 02 Oct 2018 21:40:33 GMT) CTRI Number Last Modified On 26/12/2012 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014009.4718A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0094718A1 Feldman (43) Pub. Date: (54) SYSTEMAND METHOD FORTATTOO (52) U.S. Cl. REMOVAL USPC... 601A2 (71)
(19) United States US 2014009.4718A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0094718A1 Feldman (43) Pub. Date: (54) SYSTEMAND METHOD FORTATTOO (52) U.S. Cl. REMOVAL USPC... 601A2 (71)
Fillerina. First dermo-cosmetic filler for at-home use. Innovation 2012
 First dermo-cosmetic filler for at-home use Innovation 2012 Filling wrinkles and providing greater volume for areas of the face such as the cheeckbones and lips is a topic that currently attracts a great
First dermo-cosmetic filler for at-home use Innovation 2012 Filling wrinkles and providing greater volume for areas of the face such as the cheeckbones and lips is a topic that currently attracts a great
USOO A United States Patent (19) 11 Patent Number: 6,117,843 Baroody et al. (45) Date of Patent: *Sep. 12, 2000
 USOO611.7843A United States Patent (19) 11 Patent Number: Baroody et al. () Date of Patent: *Sep. 12, 2000 54) COMPOSITIONS FOR THE TREATMENT OF No. 08/2,1, Apr. 28, 1994, Pat. No. 5,733,886, which is
USOO611.7843A United States Patent (19) 11 Patent Number: Baroody et al. () Date of Patent: *Sep. 12, 2000 54) COMPOSITIONS FOR THE TREATMENT OF No. 08/2,1, Apr. 28, 1994, Pat. No. 5,733,886, which is
MICRONEEDLING DEVICE (SKIN ROLLER) ENHANCES SKIN S ABSORPTION OF PRODUCTS
 GUNA Clinical Support Doc Dr Jo Serrentino Guna Clinical Support Line 877-486-2383 Clinicalsupport@gunacanada.com GUNA MICRONEEDLING DEVICE APPLICATIONS and TECHNIQUE MICRONEEDLING DEVICE (SKIN ROLLER)
GUNA Clinical Support Doc Dr Jo Serrentino Guna Clinical Support Line 877-486-2383 Clinicalsupport@gunacanada.com GUNA MICRONEEDLING DEVICE APPLICATIONS and TECHNIQUE MICRONEEDLING DEVICE (SKIN ROLLER)
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
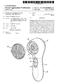 US 20140309.662A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0309662 A1 Brewer et al. (43) Pub. Date: Oct. 16, 2014 (54) EXFOLIATING BRUSH HEAD FOR A (52) U.S. Cl. PERSONAL
US 20140309.662A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0309662 A1 Brewer et al. (43) Pub. Date: Oct. 16, 2014 (54) EXFOLIATING BRUSH HEAD FOR A (52) U.S. Cl. PERSONAL
Demystifying Skin Care for Massage Therapists Chapter 5
 1 Demystifying Skin Care for Massage Therapists Chapter 5 Created by Nina Howard, Founder and Master Trainer Adapted and Edited by Kathryn Myers, CEO Bellanina Insitute BELLANINA INSTITUTE for Skin and
1 Demystifying Skin Care for Massage Therapists Chapter 5 Created by Nina Howard, Founder and Master Trainer Adapted and Edited by Kathryn Myers, CEO Bellanina Insitute BELLANINA INSTITUTE for Skin and
A natural, cost-efficient O/W emulsifier with excellent performance
 Technical Information TEGO Care PSC 3 A natural, cost-efficient O/W emulsifier with excellent performance Intended use O/W emulsifier Benefits at a glance Completely based on natural raw materials Suitable
Technical Information TEGO Care PSC 3 A natural, cost-efficient O/W emulsifier with excellent performance Intended use O/W emulsifier Benefits at a glance Completely based on natural raw materials Suitable
United States Patent (19)
 United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
SKIN BRIGHTENING SYSTEM THE POWER OF FOAM AND PHOTODAMAGE O F T H E FA C E. Powerful 3-Piece Regimen Kit
 SKIN BRIGHTENING SYSTEM THE R E V O L U T I O N A RY POWER OF FOAM C L I N I C A L LY P R O V E N T O T R E AT P I G M E N TAT I O N AND PHOTODAMAGE O F T H E FA C E Powerful 3-Piece Regimen Kit Foam drug
SKIN BRIGHTENING SYSTEM THE R E V O L U T I O N A RY POWER OF FOAM C L I N I C A L LY P R O V E N T O T R E AT P I G M E N TAT I O N AND PHOTODAMAGE O F T H E FA C E Powerful 3-Piece Regimen Kit Foam drug
reflect the beauty of science.
 reinventing the clinical skincare experience The skinbetter science team of aesthetic experts, developed Restylane and Dysport. We have a deep understanding of skin aging and what it takes to help defy
reinventing the clinical skincare experience The skinbetter science team of aesthetic experts, developed Restylane and Dysport. We have a deep understanding of skin aging and what it takes to help defy
PERSONAL CARE. INNOVATIVE & NATURAL Functional ingredients based on sugar chemistry
 PERSONAL CARE INNOVATIVE & NATURAL Functional ingredients based on sugar chemistry PERSONA INNOVATIVE & NATURAL Functional ingredients based on sugar chemistry L CARE Pack size Ecocert approved Ecocert
PERSONAL CARE INNOVATIVE & NATURAL Functional ingredients based on sugar chemistry PERSONA INNOVATIVE & NATURAL Functional ingredients based on sugar chemistry L CARE Pack size Ecocert approved Ecocert
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 2006.0024342A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0024342 A1 Bartholomew (43) Pub. Date: (54) CUSTOMIZED RETAL POINT OF SALE Related U.S. Application Data
US 2006.0024342A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0024342 A1 Bartholomew (43) Pub. Date: (54) CUSTOMIZED RETAL POINT OF SALE Related U.S. Application Data
(12) (10) Patent No.: US 6,971,424 B1. Angevine (45) Date of Patent: Dec. 6, (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi...
 United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
(19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
Supplemental January 2009
 Supplemental January 2009 Editor s note: The Surfactant Spectator is always looking for articles that are of interest to our readers. No topic is more interesting to our readers than green surfactants,
Supplemental January 2009 Editor s note: The Surfactant Spectator is always looking for articles that are of interest to our readers. No topic is more interesting to our readers than green surfactants,
Head 2 Toe (h2t) Pumpkin Peel
 Types of Pumpkin Peel Products Offered Professional 30% AHA 25oz Retail Multi-Fruit Complex/AHA 2oz Head 2 Toe (h2t) Pumpkin Peel H2t 30% AHA formula adds Lactic Acid, White Willow Bark (chamomile or yarrow
Types of Pumpkin Peel Products Offered Professional 30% AHA 25oz Retail Multi-Fruit Complex/AHA 2oz Head 2 Toe (h2t) Pumpkin Peel H2t 30% AHA formula adds Lactic Acid, White Willow Bark (chamomile or yarrow
Partners in advancing the commitment to healthy, beautiful skin. Skin Care Catalog
 Partners in advancing the commitment to healthy, beautiful skin Skin Care Catalog WHY TOPIX? Partners in advancing the commitment to healthy, beautiful skin For over 30 years, Topix Pharmaceuticals Inc.
Partners in advancing the commitment to healthy, beautiful skin Skin Care Catalog WHY TOPIX? Partners in advancing the commitment to healthy, beautiful skin For over 30 years, Topix Pharmaceuticals Inc.
Topical Skin Care L O O K, F E E L A N D L I V E B E T T E R
 L O O K, F E E L A N D L I V E B E T T E R Topical Skin Care Pycnogenol in Topical Skin Care Pycnogenol is widely used in topical and oral applications for various dermatological indications. A unique
L O O K, F E E L A N D L I V E B E T T E R Topical Skin Care Pycnogenol in Topical Skin Care Pycnogenol is widely used in topical and oral applications for various dermatological indications. A unique
Hydrolyzed Jojoba Esters to Potentiate Glycerin Moisturization
 Hydrolyzed Jojoba Esters to Potentiate Glycerin Moisturization Tiffany N. Oliphant and Douglas W. Gilmore Floratech, Chandler, AZ, USA Robert A. Harper, PhD Harper and Associates, La Jolla, CA USA KEY
Hydrolyzed Jojoba Esters to Potentiate Glycerin Moisturization Tiffany N. Oliphant and Douglas W. Gilmore Floratech, Chandler, AZ, USA Robert A. Harper, PhD Harper and Associates, La Jolla, CA USA KEY
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory OILATUM EMOLLIENT. Light Liquid Paraffin Emollient
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory OILATUM EMOLLIENT Light Liquid Paraffin Emollient QUALITATIVE AND QUANTITATIVE COMPOSITION Light Liquid Paraffin IP Base
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory OILATUM EMOLLIENT Light Liquid Paraffin Emollient QUALITATIVE AND QUANTITATIVE COMPOSITION Light Liquid Paraffin IP Base
(12) United States Patent
 (12) United States Patent DOW et al. US0017847B2 (10) Patent No.: () Date of Patent: *Feb. 11, 2003 (54) TOPICAL GEL DELIVERY SYSTEM (75) Inventors: Gordon J. Dow, Santa Rosa, CA (US); Robert W. Lathrop,
(12) United States Patent DOW et al. US0017847B2 (10) Patent No.: () Date of Patent: *Feb. 11, 2003 (54) TOPICAL GEL DELIVERY SYSTEM (75) Inventors: Gordon J. Dow, Santa Rosa, CA (US); Robert W. Lathrop,
Silsoft* A+ Technical Data Sheet. Silsoft* A+ conditioning agent
 Technical Data Sheet Silsoft* A+ Silsoft* A+ conditioning agent Description Silsoft A+ conditioning agent can help provide excellent conditioning to damaged hair. Silsoft A+ conditioning agent is a surfactant-free
Technical Data Sheet Silsoft* A+ Silsoft* A+ conditioning agent Description Silsoft A+ conditioning agent can help provide excellent conditioning to damaged hair. Silsoft A+ conditioning agent is a surfactant-free
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 2008011 6236A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0116236A1 NICKELS (43) Pub. Date: May 22, 2008 (54) COMBINATION UMBRELLA, SUPPORTAND Publication Classification
(19) United States US 2008011 6236A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0116236A1 NICKELS (43) Pub. Date: May 22, 2008 (54) COMBINATION UMBRELLA, SUPPORTAND Publication Classification
AHCare. Have younger looking skin the mild way. Amphoteric Hydroxy Complexes: all the benefits of Alpha Hydroxy Acids with enhanced tolerance
 AHCare AHCare Amphoteric Hydroxy Complexes: all the benefits of Alpha Hydroxy Acids with enhanced tolerance - "Time Release" mechanism prevents irritation, - suitable even for sensitive skin (clinical
AHCare AHCare Amphoteric Hydroxy Complexes: all the benefits of Alpha Hydroxy Acids with enhanced tolerance - "Time Release" mechanism prevents irritation, - suitable even for sensitive skin (clinical
EpiCeram Topical therapeutic Skin Barrier Emulsion
 EpiCeram Topical therapeutic Skin Barrier Emulsion PEDIAPHARM INC. Date of preparation: August 31, 2010 Summary Product Information: EpiCeram Skin Barrier Emulsion is a steroid-free, fragrance - free,
EpiCeram Topical therapeutic Skin Barrier Emulsion PEDIAPHARM INC. Date of preparation: August 31, 2010 Summary Product Information: EpiCeram Skin Barrier Emulsion is a steroid-free, fragrance - free,
LaraCare A200 Your Multi-Functional Larch Tree Active
 Personal Care Consumer Care LaraCare A200 Your Multi-Functional Larch Tree Active INCI Name: Galactoarabinan SAP #: 179796 Key Product Attributes: Reduce Transepidermal Water Loss Reduce appearance of
Personal Care Consumer Care LaraCare A200 Your Multi-Functional Larch Tree Active INCI Name: Galactoarabinan SAP #: 179796 Key Product Attributes: Reduce Transepidermal Water Loss Reduce appearance of
United States Patent (19)
 United States Patent (19) Lachapelle (54 PROCESS FOR PREPARING A PAPER PULP USING CARBON DOXDE AS AN ACDIFYINGAGENT FOR A BLEACHED PULP (75. Inventor: Raymond C. Lachapelle, Kirkland, Canada 73 Assignee:
United States Patent (19) Lachapelle (54 PROCESS FOR PREPARING A PAPER PULP USING CARBON DOXDE AS AN ACDIFYINGAGENT FOR A BLEACHED PULP (75. Inventor: Raymond C. Lachapelle, Kirkland, Canada 73 Assignee:
Gafquat 440, 755N, 755N-P, 755N-O and HS-100, HS-100-O polymers Cationic conditioning copolymers
 PRODUCT DATA Consumer Specialties ashland.com NUMBER 4817-1 (Supersedes 4817) Page 1 of 8 Gafquat 440, 755N, 755N-P, 755N-O and HS-100, HS-100-O polymers Cationic conditioning copolymers Introduction Gafquat
PRODUCT DATA Consumer Specialties ashland.com NUMBER 4817-1 (Supersedes 4817) Page 1 of 8 Gafquat 440, 755N, 755N-P, 755N-O and HS-100, HS-100-O polymers Cationic conditioning copolymers Introduction Gafquat
Types of Exfoliation MARIE PIANTINO
 Types of Exfoliation MARIE PIANTINO PEELS Skin Resurfacing for beauty has been around for thousands of years. Ancient Egyptian recipes used fruit acids combined with skin irritants Rather deep peel clients
Types of Exfoliation MARIE PIANTINO PEELS Skin Resurfacing for beauty has been around for thousands of years. Ancient Egyptian recipes used fruit acids combined with skin irritants Rather deep peel clients
