(12) United States Patent
|
|
|
- Norman Watkins
- 5 years ago
- Views:
Transcription
1 US B2 (12) United States Patent Graeber et al. (10) Patent No.: (45) Date of Patent: *Jul. 12, 2016 (54) ADMINISTRATION OF 63-(1-ADAMANTYL)-4-METHOXYPHENYL 2-NAPHTHOIC ACID FOR THE TREATMENT OF DERMATOLOGICAL DISORDERS (71) Applicant: GALDERMA RESEARCH & DEVELOPMENT, Biot (FR) (72) Inventors: Michael Graeber, Lawrenceville, NJ (US); Janusz Czernielewski, Biot (FR) (73) Assignee: GALDERMA RESEARCH & DEVELOPMENT, Biot (FR) *) Notice: Subject to any y disclaimer, the term of this patent is extended or adjusted under 35 U.S.C. 154(b) by 72 days. This patent is Subject to a terminal dis claimer. (21) Appl. No.: 14/263,758 (22) Filed: Apr. 28, 2014 (65) Prior Publication Data US 2014/ A1 Sep. 4, 2014 Related U.S. Application Data (63) Continuation of application No. 13/024,681, filed on Feb. 10, 2011, now Pat. No. 8,729,127, which is a continuation of application No. 12/902,972, filed on Oct. 12, 2010, now Pat. No. 8,703,820, which is a continuation of application No. 12/437,008, filed on May 7, 2009, now Pat. No. 7,834,060, which is a continuation of application No. 10/937,612, filed on Sep. 10, 2004, now Pat. No. 7,579,377, which is a continuation of application No. PCT/EP03/03246, filed on Mar. 12, (60) Provisional application No. 60/ , filed on Apr. 8, (30) Foreign Application Priority Data Mar. 12, 2002 (FR)... O2 O3O70 (51) Int. Cl. A6 IK3I/92 ( ) A6 IK9/00 ( ) A6 IK9/06 ( ) A6 IK3I/07 ( ) A6 IK 8/362 ( ) A61O 1704 ( ) A61 K 47/10 ( ) A61 K 47/18 ( ) A61 K 47/32 ( ) A61 K3 1/203 ( ) A61 K 47/48 ( ) (52) U.S. Cl. CPC... A61K 31/192 ( ); A61K 8/362 ( ); A61 K9/0014 ( ); A61 K9/06 ( ); A61 K3I/07 ( ); A61O 1704 ( ); A61K 3 1/203 ( ); A61 K47/10 ( ); A61 K47/183 ( ); A61 K47/32 ( ); A61 K47/48784 ( ); C07C 2103/74 ( ); Y10S 514/859 ( ) (58) Field of Classification Search CPC... A61K 31/192 See application file for complete search history. (56) References Cited U.S. PATENT DOCUMENTS 4,717,720 A 1/1988 Shroot et al. 5,098,895 A 3, 1992 Shroot et al. 5, A 5/1993 Shroot et al. RE34440 E 11/1993 Shroot et al. 5, A 4, 1994 McCook et al. 5,603,940 A 2f1997 Candau et al. 5,665,364 A 9, 1997 McAtee et al. 6,383,505 B1 5, 2002 Kaiser et al. 6,413,536 B1 7/2002 Gibson et al. 7,083,799 B1 8/2006 Giacomoni 7,579,377 B2 8, 2009 Graeber et al. 7,642,288 B2 1/2010 Graeber O A1 2005/ A1 8, 2003 Eini et al. 3/2005 Graeber et al. FOREIGN PATENT DOCUMENTS EP O B1 10, 1986 EP O A1 3, 1996 EP O B1 3, 1996 FR A 8, 1996 WO WO WOO1,28552 WOO2, A1 4/ , 2002 OTHER PUBLICATIONS Topical Formulary for CarbopolR Polymers', Noveon, Inc., The Specialty Chemicals Innovator, Pharmaceutical Polymers, pp. 1-25, Jan. 2002, Cleveland, Ohio. Body Cream, PhytosanTM, Formula No Jamoulle et al., Follicular Penetration and Distribution of Topically Applied CD 271, a new Naphthoic Acid Derivative Intended for Topical Acne Treatment. Journal of Investigative Dermatology, (1990), 94(5), , published by Nature Publishing Group. Alec et al., Skin distribution and pharmaceutical aspects of adapalene gel. J. Am. Acad. Dermatol. (1997) 36:S119-S125, pub lished by American Academy of Dermatology, Inc., US. (Continued) Primary Examiner Samira Jean-Louis (74) Attorney, Agent, or Firm Dentons US LLP (57) ABSTRACT Dermatological disorders having an inflammatory or prolif erative component are treated with pharmaceutical composi tions containing on the order of 0.3% by weight of 6-3-(1- adamantyl)-4-methoxyphenyl-2-naphthanoic acid (adapalene) or salt thereof, formulated into pharmaceutically acceptable media therefor, advantageously topically appli cable gels, creams or lotions. 21 Claims, 3 Drawing Sheets
2 Page 2 (56) References Cited OTHER PUBLICATIONS Shroot et al., A New Concept of Drug Delivery for Acne', Derma tology (1998), 196: , published by S. Karger AG, Switzer land. Rolland et al., Site-specific Drug Delivery to Pilosebaceous Struc tures Using Polymeric Microspheres'. Pharmaceutical Research (1993), vol. 10, No. 12, , published by Plenum Publishing Corporation. Alirezaietal. Etude Comparative de l'efficacité et dela tolérance de gels d'adapalene a 0.1 et 0.03 p 100 et d'un gel detrétimoine á0,025 p. 100 dans le traitement de l'acné' (Efficacy and safety comparison study of 0.1 p. 100 and 0.03 p. 100 adapalene gels and tretinoingel in the topical treatment of acne), Ann. Dermatol. Venereol. (1996), 123: , published by Paris Masson, Paris, France, with lengthy English-language Summary. Healy et al. "Acnevulgaris. British Medical Journal, 1994 vol. 308, Iss. 6932, pp Czernielewski et al. "Adapalene biochemistry and the evolution of a new topical retinoid for treatment of acne'. Journal of European Academy of Dermatology and Venerology, Dec. 2001, vol. 15, Supplement 3, pp Goldfarb et al. Photographic assessment of the effects of adapalene 0.1% and 0.3% gels and vehicle in photodamaged skin. Abstract published in European Academy of Dermatology and Venereology JEADV (2000) 14 (Suppl. 1), 315, Wiley-Blackwell, Hoboken, New Jersey. Kang et al. "Assessment of adapalene gel for the treatment of actinic keratoses and lentigines: A randomized trial. J. Am. Acad. Dermatol., Jul. 2003, pp , American Academy of Dermatology, Inc., Schaumberg, Illinois. Talukdar et al., Journal of Pharmaceutical Sciences, May 1996, vol. 85, No. 5, Sepicontrol A5, Aug. 2001, pp Differin Gel Data Sheet, available as of Nov. 1998, pp International Search Report for corresponding PCT/EP03/03246 (WO 03/ A1) in English. European Search Report for corresponding EP (in English). Minutes for Oral Proceedings for European Application EP corresponding to grandparent U.S. Appl. No. 10/937,612. File History of U.S. Pat. No. 5,098,895 Shroot et al. File History of Reissue 34,440, Shroot et al. Protest Under 37 C.F.R.S regarding U.S. Appl. No. 1 1/ (US 2007/ ) with attachments dated Jun. 14, Notification of Non-Entry of Protest, dated Aug. 29, 2008 in U.S. Applin. No. 1 1/ , filed Jul. 28, 2006.
3 U.S. Patent Jul. 12, 2016 Sheet 1 of 3 Figure 1. Regression of total lesions O 3. O % Week
4 U.S. Patent Jul. 12, 2016 Sheet 2 of 3 Figure 2. Regression of inflammatory lesions 2. O s O.30% -e-o. 10% -40 A-Ven. week
5 U.S. Patent Jul. 12, 2016 Sheet 3 of 3 Figure 3 Regression of non-inflammatory lesions O - 1 O O.30% -30 al-o. 10% -A Vet, -40-5O
6 1. ADMINISTRATION OF 63-(1-ADAMANTYL)-4-METHOXYPHENYL 2-NAPHTHOIC ACID FOR THE TREATMENT OF DERMATOLOGICAL DISORDERS CROSS-REFERENCE TO EARLIER APPLICATIONS This application is a continuation of earlier U.S. patent application Ser. No. 13/024,681, filed Feb. 10, 2011, now allowed, which is a continuation of U.S. patent application Ser. No. 12/902,972, filed Oct. 12, 2010, which is a continu ation of U.S. application Ser. No. 12/437,008, filed May 7, 2009, now U.S. Pat. No. 7,834,060, which is a continuation of earlier U.S. patent application No. 10/937,612, filed Sep.10, 2004, now U.S. Pat. No. 7,579,377, which is a continuation of PCT/EP03/03246 filed Mar. 12, 2003, and designating the United States (published in English on Sep. 18, 2003 as WO 03/ A1), which claims benefit of U.S. Provisional Application No. 60/ , filed Apr. 8, 2002, and also claims priority under 35 U.S.C. S 119 of FR-02/03070, filed Mar. 12, 2002, each earlier application being hereby expressly incorporated by reference and each assigned to the assignee hereof. CROSS REFERENCE TO OTHER RELATED APPLICATIONS See also U.S. patent application Ser. No. 12/103,182, filed Apr. 15, 2008, now U.S. Pat. No. 7,838,558, which is a divisional application of earlier filed U.S. patent application Ser. No. 10/937,612, filed Sep. 10, 2004, now U.S. Pat. No , and claims the same domestic and foreign priority as claimed herein; and U.S. patent application Ser. No. 12/772,861, filed May 3, 2010, now U.S. Pat. 7,868,044, which is a division of U.S. patent application Ser. No. 1 1/ , filed Jul. 28, 2006, now U.S. Pat. 7,737,181, which is a continuation-in-part of earlier filed U.S. patent application Ser. No. 10/937,612 filed Sep. 10, 2004, now U.S. Pat. No , and claims the same domestic and foreign priority as claimed herein; both of said applications also expressly incorporated by reference herein and assigned to the assignee hereof. BACKGROUND OF THE INVENTION 1.Technical Field of the Invention The present invention relates to the administration to indi viduals in need of such treatment of 6-3-(1-adamantyl)-4- methoxyphenyl-2-naphthanoic acid, the chemical structure of which is as follows: CHO COOH in pharmaceutical compositions, in particular dermatological compositions, for the treatment of dermatological ailments/ afflictions having an inflammatory or proliferative compo nent. 2. Description of Background and/or Related and/or Prior Art 6-3-(1-Adamantyl)-4-methoxyphenyl-2-naphthanoic acid (hereinafter referred to as adapalene) is a retinoid derived from naphtholic acid, having anti-inflammatory properties. This molecule has been the subject of development for the topical treatment of common acne and dermatoses sensitive to retinoids. Adapalene is described in EP-0, , and a process for synthesizing same is described in EP-0,358,574, both assigned to the assignee hereof. The assignee hereof markets adapalene formulated at a weight concentration of 0.1% in the form of an alcoholic lotion, an aqueous gel and a cream. These compositions are suited for the treatment of acne. Finally, adapalene is described as having a beneficial action on photodamaged skin (Photographic assessment of the effects of adapalene 0.1% and 0.3% gels and vehicle on photodamaged skin. M. Goldfarb et al., Clinical Dermatol ogy, Vienna, Austria, May 2000). SUMMARY OF THE INVENTION Novel pharmaceutical compositions have now been devel oped containing adapalene at a weight concentration of 0.3% formulated into pharmaceutically acceptable media therefor, useful for the treatment (regime or regimen) of dermatologi cal ailments, conditions or afflictions having an inflammatory or proliferative component. Specifically, it has now Surpris ingly been shown that, in addition to exhibiting better thera peutic efficacy compared to known compositions, the com positions according to the invention exhibits good tolerance, comparable to those of the known compositions with a lower concentration of active principle. The results regarding tolerance observed in trials relating to photo-damaged skin (indication photodamage'), obtained on individuals on average 65 years old, could not be exploited in the context of the present invention. Specifically, as regards use of adapalene on young individuals (in particu lar regarding acne with populations of teenagers or young adults), the skin exhibits very different physiopathological characteristics (presence of many lesions, in particular inflammatory lesions, modifying skin permeability, hyper cornification of the follicular channel, immuno response, bac terial colonization of the skin (P. acnes), sebaceous hyperpla sia with hyperseborrhea). BRIEF DESCRIPTION OF THE DRAWINGS FIG. 1 is a graph illustrating regression in the number of total lesions for 0.30% adapalene gel, as compared to 0.10% adapalene gel and the gel Vehicle without active ingredient, over a 12-week treatment period in groups of patients suffer ing from acne. FIG. 2 is a graph illustrating regression in the number of inflammatory lesions for 0.30% adapalene gel, as compared to 0.10% adapalene gel and the gel vehicle without active ingredient, over a 12-week treatment period in groups of patients Suffering from acne. FIG. 3 is a graph illustrating regression in the number of non-inflammatory lesions for 0.30% adapalene gel, as com pared to 0.10% adapalene gel and the gel vehicle without
7 3 active ingredient, over a 12-week treatment period in groups of patients suffering from acne. DETAILED DESCRIPTION OF BEST MODE AND SPECIFICAPREFERRED EMBODIMENTS OF THE INVENTION Thus, the present invention features formulating 6-3-(1- adamantyl)-4-methoxyphenyl-2-naphthanoic acid (ada palene), or its salts, into pharmaceutical compositions useful for the treatment of dermatological ailments, conditions or afflictions having an inflammatory or proliferative compo nent, such pharmaceutical compositions comprising 0.3% by weight of adapalene relative to the total weight of the com position. The term adapalene salts' is intended to mean the salts formed with a pharmaceutically acceptable base, in particular organic bases such as sodium hydroxide, potassium hydrox ide and aqueous ammonia, or organic bases such as lysine, arginine or N-methylglucamine. The term adapalene salts' is also intended to mean the salts formed with fatty amines such as dioctylamine and Stearylamine. The administration of the compositions according to the invention may be carried out enterally, parenterally, topically or occularly. The pharmaceutical compositions according to the inven tion are preferably administered topically. Enterally, the pharmaceutical composition may be in the form of tablets, gelatin capsules, dragées, syrups, suspen sions, solutions, powders, granules, emulsions, or Suspen sions of microspheres or nanospheres or of lipid or polymeric vesicles for controlled release. Parenterally, the pharmaceu tical composition may be in the form of Solutions or Suspen sions for infusion or for injection. Topically, the pharmaceutical compositions according to the invention are more particularly suited for treatment of the skin and the mucous membranes, and may be in the form of ointments, creams, milks, pomades, powders, impregnated pads, Solutions, gels, sprays, lotions or Suspensions. They may also be in the form of Suspensions of microspheres or nanospheres or of lipid or polymeric vesicles, or of polymeric patches and hydrogels for controlled release. These compo sitions for topical application may be in anhydrous form, in aqueous form or in the form of an emulsion. In a preferred embodiment of the invention, the pharma ceutical composition according to the invention is in the form of a gel, a cream or a lotion. In particular, the pharmaceutical composition may be an aqueous gel containing in particular one or more ingredients selected from among Carbomer 940 (BF Goodrich, Carbopol 980) and propylene glycol, or a cream containing in particular one or more ingredients selected from among perhy drosqualene, cyclomethicone, PEG-20 methyl glucose seq uistearate and methylglucose sequistearate, or a polyethylene glycol-based alcoholic lotion. The pharmaceutical compositions according to the inven tion may also contain inert additives or combinations of these additives, such as Wetting agents: flavor enhancers; preservatives Such as para-hydroxybenzoic acid esters; stabilizers; moisture regulators; ph regulators; osmotic pressure modifiers; emulsifiers; UV-A and UV-B screening agents; and antioxidants, such as C-tocopherol, butylhydroxyani sole or butylhydroxytoluene, Superoxide dismutase, ubiquinol or certain metal chelating agents. Of course, those skilled in the art will take care to select the optional compound(s) to be added to these compositions in Such a way that the advantageous properties intrinsically associated with the present invention are not, or are not Sub stantially, adversely affected by the envisaged addition. The formulation of adapalene into pharmaceutical compo sitions according to the invention is especially intended for the treatment of dermatological ailments, conditions and afflictions having an inflammatory or proliferative compo nent, selected from the group consisting of: common acne, comedones, polymorphous acne, nodulo cystic acne, acne conglobata, secondary acne such as Solar, drug-related or occupational acne; widespread and/or severe forms of psoriasis, ichtyoses and ichtyosiform states; Darier's disease; actinic keratoses; palmo plantar keratoderma and keratosis pilaris; leucoplasias and leucoplasiform states, lichen planus; any benign or malignant, severe and extensive dermato logical preparations. The compositions according to the invention are particu larly Suitable for the treatment of acne. Such as common acne, and in particular for the treatment of common acne of mod erate to moderately severe intensity. Various formulations of compositions comprising 0.3% of adapalene will now be given, it being understood that same are intended only as illustrative and innowise limitative. Also given are results showing the therapeutic effects of the com positions according to the invention and the good tolerance to same by the treated patients. In said examples to follow, all parts and percentages are given by weight, unless otherwise indicated. EXAMPLE 1. Formulation for Topical Administration In this example, various specific topical formulations com prising 0.3% of adapalene are illustrated. The adapalene of the present example is provided by Sylachim, Division Finorga (product reference CF ). (a) Cream: Adapalene Carbomer 934 (BF Goodrich Carbopol 974) Disodium edietate PEG methylglucose sesquistearate Methylglucose sesquistearate Glycerol Methyl paraben Cyclomethicone Perhydrosqualene Phenoxyethanol Propyl paraben Sodium hydroxide quantity required for ph 6.5 +/-0.3 Purified water 3 mg 4.5 mg 1 mg 35 mg 35 mg 30 mg 2 mg 130 mg 60 mg 5 mg 1 mg q.s. 1 g
8 (b) Lotion: Adapalene PEG 400 Ethanol (c) Aqueous Gel: Adapalene Carbomer 940 (B F Goodrich Carbopol980) Disodium edietate Methyl paraben Poloxamer 124 Propylene glycol Sodium hydroxide: amount required to obtain a ph 5.0 +f-0.3 Purified water 5 EXAMPLE 2 3 mg 700 mg q.s. 1 g 3 mg 11 mg 1 mg 2 mg 2 mg 40 mg q.s. 1 g Effectiveness of 0.3% Adapalene Gel and Comparison with the 0.1% Adapalene Gel Tests were carried out on a population consisting of patients Suffering from acne. In this population, three groups were differentiated; the first received a daily topical applica tion of the 0.3% adapalene gel, the second a daily topical application of the 0.1% adapalenegel in the same vehicle, and the third is a control group which receives a daily topical application of the gel corresponding to the composition of the first two gels but containing no active agent. FIGS. 1 to 3 provide the results obtained in terms of regres sion of the number of lesions according to their nature. These observations lead to the following conclusions: the 0.3% adapalene gel acts more rapidly than the 0.1% adapalene gel; specifically, from the fourth week of treat ment, a difference is noted between the effectiveness of the 0.1% adapalene gel and the 0.3% adapalene gel; the 0.3% adapalene gel produces a clearly greater thera peutic effect after 8 weeks of treatment. EXAMPLE 3 Tolerance Regarding the 0.3% Adapalene Gel 1. Measurement of the Plasma Concentration of Ada palene: Eight individuals suffering from common acne of medium to moderately severe intensity are treated for 10 days with 2g of 0.3% adapalene gel applied daily over 1000 cm of skin to be treated (face, chest and back). Blood samples are taken on the days 1, 2, 4, 6, 8 and 10. During day 10, and following the final application, Samples are taken at 1, 2, 6, 8, 10, 12, 16 and 24 hours. The plasma concentration of total adapalene (free and con jugated) in these samples is determined using the following protocol: enzymatic hydrolysis with a mixture of B-glucurodinase and arylsulfatase; liquid-liquid extraction; passage through HPLC (high performance liquid chroma tography); and then fluorometric detection. This method makes it possible to detect a minimum con centration of 0.15 ng/ml and permits quantification of the adapalene for a minimum concentration of 0.25 ng/ml CONCLUSION The plasma concentrations of adapalene measured after 10 days of treatment are very low and confirm the safety of daily use of the 0.3% adapalene gel. 2 a) Clinical Observation of the Side Effects Caused by Topical Administration of the 0.3% Adapalene Gel: Two types of observation could be made: firstly, monitoring of the patients treated within the frame work of point 1 of the present Example 3 made it possible to note that tolerance to the 0.3% adapalene gel was good for all patients. They all showed signs of dryness of the skin and of descuamation with a maximum on the seventh day of treat ment, these symptoms then decrease up to the end of the treatment. 2b) Furthermore, Reference may Also be Made to the Tests Described in Example 2 Above: In parallel to the measurements of effectiveness, the experimenters recorded the possible side effects caused, firstly, by topical application of the 0.3% adapalene gel and those caused, secondly, by application of the 0.1% adapalene gel; finally, the same observations were made on a control population to which a gel without active principle was admin istered. These observations are reported in the table below. Local undesirable 0.3% adapalene 0.1% adapalene Vehicle gel effects gel (N = 70) gel (N = 70) (N = 74) Skin and secondary 31 (44.3%) 28 (40.0%) 5 (6.8%) structures (nails, hair) Dry skin 16 (22.9%) 13 (18.6%) 2 (2.7%) Erythema 8 (11.4%) 3 (4.3%) 0 (0.0%) Skin discomfort 8 (11.4%) 7 (10.0%) 0 (0.0%) Desquamation 6 (8.6%) 5 (7.1%) 0 (0.0%) Dermatitis 3 (4.3%) 1 (1.4%) 0 (0.0%) Pruritus 3 (4.3%) 1 (1.4%) 1 (1.4%) rritant dermatitis 2 (2.9%) 7 (10.0%) 0 (0.0%) Local allergic reactions 1 (1.4%) 0 (0.0%) 0 (0.0%) Pediculosis 1 (1.4%) 0 (0.0%) 0 (0.0%) Contact dermatitis 1 (1.4%) 0 (0.0%) 0 (0.0%) nsolation 1 (1.4%) 3 (4.3%) 1 (1.4%) Burning sensation 1 (1.4%) 0 (0.0%) 0 (0.0%) Urticaria 11.4%) 0 (0.0%) 0 (0.0%) infection 1 (1.4%) 0 (0.0%) 0 (0.0%) Excoriation 0 (0.0%) 0 (0.0%) 1 (1.4%) Eczema 0 (0.0%) 0 (0.0%) 1 (1.4%) Oedema 0 (0.0%) 1 (1.4%) 0 (0.0%) From this table, it is noted that the occurrence of undesir able side effects is statistically the same for the two gels with the different concentrations of active agent. The intensity of the undesirable side effects is average, which leads to the conclusion that the two gels are well-tolerated by the patients. On the basis of these observations, it may be concluded that patients suffering from common acne can be treated with 0.3% adapalene gel. Such an exposure to adapalene being described as weak or very weak under clinical conditions. It therefore ensues from these various studies that a phar maceutical composition containing 0.3% of adapalene exhib its a benefit/risk ratio which makes it particularly suitable for the treatment of dermatological maladies having an inflam matory or proliferative component, and in particular, com OaCC. Each patent, patent application, publication and literature article/report cited or indicated herein is hereby expressly incorporated by reference. While the invention has been described in terms of various specific and preferred embodiments, the skilled artisan will appreciate that various modifications, Substitutions, omis
9 7 sions, and changes may be made without departing from the spirit thereof. Accordingly, it is intended that the scope of the present invention be limited solely by the scope of the fol lowing claims, including equivalents thereof. What is claimed is: 1. A method for eliciting an early onset of action in regres sion of inflammatory lesions, regression of non-inflammatory lesions or regression of total acne lesions in treating common acne afflicting an individual s skin, the individual being in need of Such treatment, comprising topically administering daily to said individual an anti-acne effective amount of a pharmaceutical composition which comprises 0.3% by weight of 6-3-(1-adamantyl)-4-methoxyphenyl-2-naph thoic acid (adapalene) or salt thereof, formulated into a phar maceutically acceptable medium therefor, said composition being a gel or a cream, wherein said early onset of action occurs by four weeks after treatment begins and is demon strated by regression of inflammatory lesions, regression of non-inflammatory lesions or regression of total acne lesions after four weeks of treatment greater than that demonstrated by Vehicle alone or by a similar composition comprising 0.1% by weight of adapalene after four weeks of treatment. 2. The method according to claim 1, wherein the common acne is of moderate to moderately severe intensity. 3. The method according to claim 1, wherein the compo sition is a gel comprising adapalene, carbomer 940, disodium edetate, methyl paraben, poloxamer 124, propylene glycol, sodium hydroxide and purified water. 4. The method according to claim 1, wherein the compo sition comprises a gel. 5. The method according to claim 2, wherein the compo sition comprises a gel. 6. A method for eliciting an early onset of action in regres sion of inflammatory lesions in treating common acne afflict ing an individual s skin, the individual being in need of Such treatment, comprising topically administering daily to said individual an anti-acne effective amount of a pharmaceutical composition which comprises 0.3% by weight of 6-3-(1- adamantyl)-4-methoxyphenyl-2-naphthoic acid (adapalene) or salt thereof, formulated into a pharmaceutically acceptable medium therefor, said composition being a gel or a cream, wherein said early onset of action occurs by four weeks after treatment begins and is demonstrated by regression of inflam matory lesions after four weeks of treatment greater than that demonstrated by Vehicle alone or by a similar composition comprising 0.1% by weight of adapalene after four weeks of treatment. 7. The method according to claim 6, wherein the common acne is of moderate to moderately severe intensity. 8. The method according to claim 6, wherein the compo sition is a gel comprising adapalene, carbomer 940, disodium edetate, methyl paraben, poloxamer 124, propylene glycol, sodium hydroxide and purified water. 9. The method according to claim 6, wherein the compo sition comprises a gel. 10. The method according to claim 7, wherein the compo sition comprises a gel A method foreliciting an early onset of action in regres sion of non-inflammatory lesions in treating common acne afflicting an individual s skin, the individual being in need of Such treatment, comprising topically administering daily to said individual an anti-acne effective amount of a pharmaceu tical composition which comprises 0.3% by weight of 6-3- (1-adamantyl)-4-methoxyphenyl-2-naphthoic acid (ada palene) or salt thereof, formulated into a pharmaceutically acceptable medium therefor, said composition being a gel or a cream, said early onset of action occurring by four weeks after treatment begins and being demonstrated by regression of non-inflammatory lesions after four weeks of treatment greater than that demonstrated by vehicle alone or by a similar composition comprising 0.1% by weight of adapalene after four weeks of treatment. 12. The method according to claim 11, wherein the com mon acne is of moderate to moderately severe intensity. 13. The method according to claim 11, wherein the com position is a gel comprising adapalene, carbomer 940, diso dium edetate, methyl paraben, poloxamer 124, propylene glycol, Sodium hyrdoxide and purified water. 14. The method according to claim 12, wherein the com position is a gel comprising adapalene, carbomer940, diso dium edetate, methyl paraben, poloxamer 124, propylene glycol, Sodium hydroxide and purified water. 15. The method according to claim 11, wherein the com position comprises a gel. 16. The method according to claim 12, wherein the com position comprises a gel. 17. A method foreliciting an early onset of action in regres sion of total acne lesions in treating common acne afflicting an individual s skin, the individual being in need of such treatment, comprising topically administering daily to said individual an anti-acne effective amount of a pharmaceutical composition which comprises 0.3% by weight of 6-3-(1- adamantyl)-4-methoxyphenyl-2-naphthoic acid (adapalene) or salt thereof, formulated into a pharmaceutically acceptable medium therefor, said composition being a gel or a cream, said early onset of action occurring by four weeks after treat ment begins and being demonstrated by regression of total acne lesions after four weeks of treatment greater than that demonstrated by Vehicle alone or by a similar composition comprising 0.1% by weight of adapalene after four weeks of treatment. 18. The method according to claim 17, wherein the com mon acne is of moderate to moderately severe intensity. 19. The method according to claim 17, wherein the com position comprises a gel. 20. The method according to claim 18, wherein the com position comprises a gel. 21. The method according to claim 17, wherein the com position is a gel comprising adapalene, carbomer 940, diso dium edetate, methyl paraben, poloxamer 124, propylene glycol, Sodium hydroxide and purified water. k k k k k
(12) United States Patent
 USOO872.9127B2 (12) United States Patent Graeber et al. (54) ADMINISTRATION OF 6-3-(1-ADAMANTYL)-4-METHOXYPHENYL 2-NAPHTHOIC ACID FOR THE TREATMENT OF DERMATOLOGICAL DISORDERS (75) Inventors: Michael Graeber,
USOO872.9127B2 (12) United States Patent Graeber et al. (54) ADMINISTRATION OF 6-3-(1-ADAMANTYL)-4-METHOXYPHENYL 2-NAPHTHOIC ACID FOR THE TREATMENT OF DERMATOLOGICAL DISORDERS (75) Inventors: Michael Graeber,
(12) United States Patent
 US008785420B2 (12) United States Patent Abou Chacra Vernet et al. (54) COMBINATION/ASSOCIATION OF ADAPALENE AND BENZOYL PEROXDE FORTREATING ACNELESIONS (75) Inventors: Marie-Line Abou Chacra Vernet, Nice
US008785420B2 (12) United States Patent Abou Chacra Vernet et al. (54) COMBINATION/ASSOCIATION OF ADAPALENE AND BENZOYL PEROXDE FORTREATING ACNELESIONS (75) Inventors: Marie-Line Abou Chacra Vernet, Nice
(12) United States Patent
 USOO8071644B2 (12) United States Patent Abou-Chacra Vernet et al. (10) Patent No.: (45) Date of Patent: *Dec. 6, 2011 (54) COMBINATIONS OF ADAPALENE AND BENZOYL PEROXDE FOR TREATING ACNE LESIONS (75) Inventors:
USOO8071644B2 (12) United States Patent Abou-Chacra Vernet et al. (10) Patent No.: (45) Date of Patent: *Dec. 6, 2011 (54) COMBINATIONS OF ADAPALENE AND BENZOYL PEROXDE FOR TREATING ACNE LESIONS (75) Inventors:
(12) United States Patent (10) Patent No.: US 6,841,523 B1
 USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0165220 A1 Chang et al. US 201701 65220A1 (43) Pub. Date: (54) (71) (72) (21) (22) (60) (60) TOPCAL PHARMACEUTICAL FORMULATIONS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0165220 A1 Chang et al. US 201701 65220A1 (43) Pub. Date: (54) (71) (72) (21) (22) (60) (60) TOPCAL PHARMACEUTICAL FORMULATIONS
(12) United States Patent
 US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
PRODUCT INFORMATION DIFFERIN ADAPALENE 0.1% TOPICAL GEL
 PRODUCT INFORMATION DIFFERIN ADAPALENE 0.1% TOPICAL GEL NAME OF THE MEDICINE DIFFERIN Topical Gel: Adapalene 1 mg/g (0.1%) Common Name: Adapalene Chemical Name: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic
PRODUCT INFORMATION DIFFERIN ADAPALENE 0.1% TOPICAL GEL NAME OF THE MEDICINE DIFFERIN Topical Gel: Adapalene 1 mg/g (0.1%) Common Name: Adapalene Chemical Name: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic
Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use
 9 October 2017 EMA/CHMP/606830/2014 Committee for Human Medicinal Products (CHMP) Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use Draft agreed by
9 October 2017 EMA/CHMP/606830/2014 Committee for Human Medicinal Products (CHMP) Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use Draft agreed by
(12) United States Patent
 US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
EpiCeram Topical therapeutic Skin Barrier Emulsion
 EpiCeram Topical therapeutic Skin Barrier Emulsion PEDIAPHARM INC. Date of preparation: August 31, 2010 Summary Product Information: EpiCeram Skin Barrier Emulsion is a steroid-free, fragrance - free,
EpiCeram Topical therapeutic Skin Barrier Emulsion PEDIAPHARM INC. Date of preparation: August 31, 2010 Summary Product Information: EpiCeram Skin Barrier Emulsion is a steroid-free, fragrance - free,
12) United States Patent 10) Patent No.: US 6,433,024 B1
 USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
PRODUCT INFORMATION DIFFERIN Adapalene 1mg/g TOPICAL CREAM
 PRODUCT INFORMATION DIFFERIN Adapalene 1mg/g TOPICAL CREAM NAME OF THE MEDICINE Common Name: Adapalene Chemical Name: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid Molecular Formula: C 28 H 28 O
PRODUCT INFORMATION DIFFERIN Adapalene 1mg/g TOPICAL CREAM NAME OF THE MEDICINE Common Name: Adapalene Chemical Name: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid Molecular Formula: C 28 H 28 O
(12) United States Patent (10) Patent No.: US 6,752,627 B2
 USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
United States Patent (19) Katz
 United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
III. United States Patent 19 Jordan 5,389,129. Feb. 14, ). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee:
 United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
(12) United States Patent (10) Patent No.: US 7434,929 B2
 US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
4.3. Contraindications Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Tactuo 0.1% / 2.5% gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of gel contains: Adapalene 1 mg (0.1 %) Benzoyl Peroxide 25 mg
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Tactuo 0.1% / 2.5% gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of gel contains: Adapalene 1 mg (0.1 %) Benzoyl Peroxide 25 mg
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
(19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
The Effects of Shear on Neutralized Carbomers in Aqueous Conditions
 The Effects of Shear on Neutralized Carbomers in Aqueous Conditions Lyndel Speedy 18/07/2014 With thanks to Ensign Laboratories 1 Abstract Carbomer is the generic name for a class of high molecular weight
The Effects of Shear on Neutralized Carbomers in Aqueous Conditions Lyndel Speedy 18/07/2014 With thanks to Ensign Laboratories 1 Abstract Carbomer is the generic name for a class of high molecular weight
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
Ref. 11. (12) Patent Application Publication (10) Pub. No.: US 2012/ A1. (19) United States. Polstein et al. (43) Pub. Date: Jun.
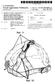 (19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(12) United States Patent (10) Patent No.: US 9.407, B2
 USOO9407742B2 (12) United States Patent (10) Patent No.: US 9.407,742 9 9 B2 NOble Nava (45) Date of Patent: Aug. 2, 2016 (54) CELL PHONE HOLSTER 2005/008 (2013.01); A45F 2200/0516 (2013.01); H04B 2001/3861
USOO9407742B2 (12) United States Patent (10) Patent No.: US 9.407,742 9 9 B2 NOble Nava (45) Date of Patent: Aug. 2, 2016 (54) CELL PHONE HOLSTER 2005/008 (2013.01); A45F 2200/0516 (2013.01); H04B 2001/3861
New Zealand Datasheet
 New Zealand Datasheet 1 PRODUCT NAME Epiduo 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% / benzoyl peroxide 2.5% gel 3 PHARMACEUTICAL FORM Epiduo is a white to very pale yellow opaque gel
New Zealand Datasheet 1 PRODUCT NAME Epiduo 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% / benzoyl peroxide 2.5% gel 3 PHARMACEUTICAL FORM Epiduo is a white to very pale yellow opaque gel
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
(12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001
 US006257248B1 (12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001 (54) BOTH HAND HAIR CUTTING METHOD 5,991,918 * 11/1999 Choate..... 2/21 6,079,107 * 6/2000
US006257248B1 (12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001 (54) BOTH HAND HAIR CUTTING METHOD 5,991,918 * 11/1999 Choate..... 2/21 6,079,107 * 6/2000
(12) United States Patent (10) Patent No.: US 6,308,717 B1
 USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
The solution contains isopropyl alcohol 50% v/v, propylene glycol, and water.
 Cleocin T (clindamycin phosphate topical solution, USP) (clindamycin phosphate topical gel) (clindamycin phosphate topical lotion) For External Use DESCRIPTION CLEOCIN T Topical Solution and CLEOCIN T
Cleocin T (clindamycin phosphate topical solution, USP) (clindamycin phosphate topical gel) (clindamycin phosphate topical lotion) For External Use DESCRIPTION CLEOCIN T Topical Solution and CLEOCIN T
(12) United States Patent
 USOO8445.543B2 (12) United States Patent Abou-Chacra Vernet et al. (54) COMBINATIONS OF ADAPALENE AND BENZOYL PEROXDE FOR TREATING ACNE LESIONS (75) Inventors: Marie-line Abou-Chacra Vernet, Nice (FR);
USOO8445.543B2 (12) United States Patent Abou-Chacra Vernet et al. (54) COMBINATIONS OF ADAPALENE AND BENZOYL PEROXDE FOR TREATING ACNE LESIONS (75) Inventors: Marie-line Abou-Chacra Vernet, Nice (FR);
EPIDUO GEL PRODUCT INFORMATION
 EPIDUO GEL PRODUCT INFORMATION NAME OF THE MEDICINE EPIDUO Topical gel: 0.1% adapalene + 2.5% benzoyl peroxide Common Names: Adapalene and benzoyl peroxide Chemical Name : - Adapalene: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic
EPIDUO GEL PRODUCT INFORMATION NAME OF THE MEDICINE EPIDUO Topical gel: 0.1% adapalene + 2.5% benzoyl peroxide Common Names: Adapalene and benzoyl peroxide Chemical Name : - Adapalene: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014009.4718A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0094718A1 Feldman (43) Pub. Date: (54) SYSTEMAND METHOD FORTATTOO (52) U.S. Cl. REMOVAL USPC... 601A2 (71)
(19) United States US 2014009.4718A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0094718A1 Feldman (43) Pub. Date: (54) SYSTEMAND METHOD FORTATTOO (52) U.S. Cl. REMOVAL USPC... 601A2 (71)
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
New Zealand Datasheet. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% topical gel
 New Zealand Datasheet 1 PRODUCT NAME DIFFERIN 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% topical gel 3 PHARMACEUTICAL FORM DIFFERIN topical gel is a smooth white gel containing 1 mg/g adapalene.
New Zealand Datasheet 1 PRODUCT NAME DIFFERIN 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% topical gel 3 PHARMACEUTICAL FORM DIFFERIN topical gel is a smooth white gel containing 1 mg/g adapalene.
(12) United States Patent (10) Patent No.: US 6,413,305 B1
 USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O130248A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0130248A1 MOZZOne et al. (43) Pub. Date: (54) TOPICAL ANTI-INFLAMMATORY COMPOSITIONS (76) Inventors: Keith
(19) United States US 2003O130248A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0130248A1 MOZZOne et al. (43) Pub. Date: (54) TOPICAL ANTI-INFLAMMATORY COMPOSITIONS (76) Inventors: Keith
Topical Steroid Therapy. Shireen Velangi Consultant Dermatology Queen Elizabeth Hospital Birmingham UK
 Topical Steroid Therapy Shireen Velangi Consultant Dermatology Queen Elizabeth Hospital Birmingham UK Aim of the Workshop Non Dermatologists (Dermatologists should go to the Professors panel now!) To increase
Topical Steroid Therapy Shireen Velangi Consultant Dermatology Queen Elizabeth Hospital Birmingham UK Aim of the Workshop Non Dermatologists (Dermatologists should go to the Professors panel now!) To increase
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Epiduo 0.3% / 2.5% gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of gel contains: adapalene 3 mg (0.3%) benzoyl peroxide 25
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Epiduo 0.3% / 2.5% gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of gel contains: adapalene 3 mg (0.3%) benzoyl peroxide 25
United States Patent (19) Humbrecht
 United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 TOPICAL RETINOID AND COMBINATION PRODUCTS: ATRALIN (tretinoin) gel AVITA (tretinoin) cream and gel DIFFERIN (adapalene) cream, gel, lotion (Over-the-Counter Differin is a plan exclusion) EPIDUO (adapalene-benzoyl
TOPICAL RETINOID AND COMBINATION PRODUCTS: ATRALIN (tretinoin) gel AVITA (tretinoin) cream and gel DIFFERIN (adapalene) cream, gel, lotion (Over-the-Counter Differin is a plan exclusion) EPIDUO (adapalene-benzoyl
(12) United States Patent (10) Patent No.: US 7,585,200 B1
 US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985
 United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985 54 INK RIBBON CASSETTE PROVIDED WITH 56) References Cited AN EMPREGNATION DEVICE U.S. PATENT DOCUMENTS s 2,76,539
United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985 54 INK RIBBON CASSETTE PROVIDED WITH 56) References Cited AN EMPREGNATION DEVICE U.S. PATENT DOCUMENTS s 2,76,539
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
(19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
(12) (10) Patent No.: US 6,971,424 B1. Angevine (45) Date of Patent: Dec. 6, (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi...
 United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
WWWWW. ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / A1. 19 United States
 THE MAIN TEA ETA AITOR A TT MA N ALUMINIUM TIN US 20170266826A1 19 United States ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / 0266826 A1 Kole et al. ( 43 ) Pub. Date : Sep. 21, 2017
THE MAIN TEA ETA AITOR A TT MA N ALUMINIUM TIN US 20170266826A1 19 United States ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / 0266826 A1 Kole et al. ( 43 ) Pub. Date : Sep. 21, 2017
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(12) United States Patent (10) Patent No.: US 6,422,036 B1. Giannis et al. (45) Date of Patent: Jul. 23, 2002
 USOO6422036B1 (12) United States Patent (10) Patent No.: US 6,422,036 B1 Giannis et al. (45) Date of Patent: Jul. 23, 2002 (54) JEWELRY CLASP 4,611,368 9/1986 Battersby... 24/116 R 5,214,940 A * 6/1993
USOO6422036B1 (12) United States Patent (10) Patent No.: US 6,422,036 B1 Giannis et al. (45) Date of Patent: Jul. 23, 2002 (54) JEWELRY CLASP 4,611,368 9/1986 Battersby... 24/116 R 5,214,940 A * 6/1993
Package leaflet: Information for the user. ZORAC 0.05% gel ZORAC 0.1% gel. Tazarotene
 Package leaflet: Information for the user ZORAC 0.05% gel ZORAC 0.1% gel Tazarotene Read all of this leaflet carefully before you start using this medicine because it contains important information for
Package leaflet: Information for the user ZORAC 0.05% gel ZORAC 0.1% gel Tazarotene Read all of this leaflet carefully before you start using this medicine because it contains important information for
STAGES OF PHARMACEUTICAL MANUFACTURING
 STAGES OF PHARMACEUTICAL MANUFACTURING API Finished Product API Primary Packaging Secondary Packaging Excipients Starting Materials (Chemicals) 1 PHARMACEUTICAL MANUFACTURING OF NORMAL DOSAGE FORMS 2 Dosage
STAGES OF PHARMACEUTICAL MANUFACTURING API Finished Product API Primary Packaging Secondary Packaging Excipients Starting Materials (Chemicals) 1 PHARMACEUTICAL MANUFACTURING OF NORMAL DOSAGE FORMS 2 Dosage
United States Patent (19)
 United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
(19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
Int. Cl."... F21V1/06 U.S. C /352; 362/358. References Cited U.S. PATENT DOCUMENTS 3,787,676 l/1974 Korach /352
 United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory NEDAX -5 LOTION. Permethrin Lotion 5% w/w
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory NEDAX -5 LOTION Permethrin Lotion 5% w/w QUALITATIVE AND QUANTITATIVE COMPOSITION Permethrin 5% w/w in a aqueous base
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory NEDAX -5 LOTION Permethrin Lotion 5% w/w QUALITATIVE AND QUANTITATIVE COMPOSITION Permethrin 5% w/w in a aqueous base
Package leaflet: Information for the patient. Epiduo 0.3% / 2.5% gel adapalene / benzoyl peroxide
 Package leaflet: Information for the patient Epiduo 0.3% / 2.5% gel adapalene / benzoyl peroxide Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the patient Epiduo 0.3% / 2.5% gel adapalene / benzoyl peroxide Read all of this leaflet carefully before you start using this medicine because it contains important information
The secondary objective was to evaluate the cosmetic properties and its efficacy after 28 days.
 P0090 CLINICAL EVALUATION OF CUTANEOUS SAFETY AND EFFICACY OF A FACE EMULSION CONTAINING MYRTACIN EXTRACT, PP VITAMIN ANS SABAL EXTRACT ON ACNE PRONE SKIN SUBJECTS TREATED BY TOPICAL ANTI ACNE DRUG THERAPY.
P0090 CLINICAL EVALUATION OF CUTANEOUS SAFETY AND EFFICACY OF A FACE EMULSION CONTAINING MYRTACIN EXTRACT, PP VITAMIN ANS SABAL EXTRACT ON ACNE PRONE SKIN SUBJECTS TREATED BY TOPICAL ANTI ACNE DRUG THERAPY.
(12) United States Patent (10) Patent No.: US 9, B2
 USOO9566228B2 (12) United States Patent (10) Patent No.: US 9,566.228 B2 Jeong (45) Date of Patent: Feb. 14, 2017 (54) BUBBLE TYPE WATERLESS SHAMPOO (2013.01); A61K 8/442 (2013.01); A61K 8/64 COMPOSITION
USOO9566228B2 (12) United States Patent (10) Patent No.: US 9,566.228 B2 Jeong (45) Date of Patent: Feb. 14, 2017 (54) BUBBLE TYPE WATERLESS SHAMPOO (2013.01); A61K 8/442 (2013.01); A61K 8/64 COMPOSITION
TAGRAVIT TM R1 Encapsulated pure retinol. March 2015
 TAGRAVIT TM R1 Encapsulated pure retinol March 2015 Retinol- Background Retinol is a form of Vitamin A having a small molecular structure with MW of 286.45 g/mol. Retinol can penetrate the outer layers
TAGRAVIT TM R1 Encapsulated pure retinol March 2015 Retinol- Background Retinol is a form of Vitamin A having a small molecular structure with MW of 286.45 g/mol. Retinol can penetrate the outer layers
(12) United States Patent
 (12) United States Patent USOO7374282B2 (10) Patent No.: US 7,374.282 B2 Tendler (45) Date of Patent: May 20, 2008 (54) METHOD AND APPARATUS FOR VIEWING 6,623,116 B2 * 9/2003 Kerns et al.... 351,165 POLARIZED
(12) United States Patent USOO7374282B2 (10) Patent No.: US 7,374.282 B2 Tendler (45) Date of Patent: May 20, 2008 (54) METHOD AND APPARATUS FOR VIEWING 6,623,116 B2 * 9/2003 Kerns et al.... 351,165 POLARIZED
(12) United States Patent (10) Patent No.: US 9,517,183 B2
 USO0951.7183B2 (12) United States Patent (10) Patent No.: Ward (45) Date of Patent: Dec. 13, 2016 (54) NIPPLE ABRASION PROTECTOR 4.333,471 A 6, 1982 Nakai 4,640,288 A 2, 1987 Hattori (71) Applicant: Patrick
USO0951.7183B2 (12) United States Patent (10) Patent No.: Ward (45) Date of Patent: Dec. 13, 2016 (54) NIPPLE ABRASION PROTECTOR 4.333,471 A 6, 1982 Nakai 4,640,288 A 2, 1987 Hattori (71) Applicant: Patrick
Reference ID: CONTRAINDICATIONS None. (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use EPIDUO Gel safely and effectively. See full prescribing information for EPIDUO Gel. EPIDUO (adapalene
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use EPIDUO Gel safely and effectively. See full prescribing information for EPIDUO Gel. EPIDUO (adapalene
(12) United States Patent
 USOO8663699B2 (12) United States Patent Chang et al. (54) TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING ALOW CONCENTRATION OF BENZOYL PEROXDE IN SUSPENSION IN WATER ANDA WATER-MSCIBLE ORGANIC SOLVENT
USOO8663699B2 (12) United States Patent Chang et al. (54) TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING ALOW CONCENTRATION OF BENZOYL PEROXDE IN SUSPENSION IN WATER ANDA WATER-MSCIBLE ORGANIC SOLVENT
Management of acne requires proper application
 DRUG THERAPY TOPICS A Qualitative and Quantitative Assessment of the Application and Use of Topical Acne Medication by Patients James Q. Del Rosso, DO Management of acne requires proper application of
DRUG THERAPY TOPICS A Qualitative and Quantitative Assessment of the Application and Use of Topical Acne Medication by Patients James Q. Del Rosso, DO Management of acne requires proper application of
PDF of Trial CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Tue, 02 Oct 2018 21:40:33 GMT) CTRI Number Last Modified On 26/12/2012 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Tue, 02 Oct 2018 21:40:33 GMT) CTRI Number Last Modified On 26/12/2012 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
(12) United States Patent (10) Patent No.: US 6,582,709 B1
 USOO82709B1 (12) United States Patent (10) Patent No.: Maor et al. () Date of Patent: Jun. 24, 2003 (54) CREAM COMPOSITION COMPRISING (56) References Cited DEAD SEA MUD U.S. PATENT DOCUMENTS (75) Inventors:
USOO82709B1 (12) United States Patent (10) Patent No.: Maor et al. () Date of Patent: Jun. 24, 2003 (54) CREAM COMPOSITION COMPRISING (56) References Cited DEAD SEA MUD U.S. PATENT DOCUMENTS (75) Inventors:
Children s Hospital Of Wisconsin
 Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
(12) United States Patent
 (12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
(12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes
 Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes Chapter 18 (Viscosity-inducing Agents) 1. What is Carbomer NF? 2. Carbomer is commonly referred to
Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes Chapter 18 (Viscosity-inducing Agents) 1. What is Carbomer NF? 2. Carbomer is commonly referred to
United States Patent (19) Schunter
 United States Patent (19) Schunter 11 45 US005699555A Patent Number: Date of Patent: Dec. 23, 1997 54 CHILD'S WAISTBELTAND LEASH FOR PROTECTIONAGAINSTABDUCTION OF A CHLD 76 Inventor: Christine K. Schunter,
United States Patent (19) Schunter 11 45 US005699555A Patent Number: Date of Patent: Dec. 23, 1997 54 CHILD'S WAISTBELTAND LEASH FOR PROTECTIONAGAINSTABDUCTION OF A CHLD 76 Inventor: Christine K. Schunter,
A novel daily moisturizing cream for effective management of mild to moderate Atopic Dermatitis in infants and children
 TM Weber PhD 1, F Samarin MD 3, M Babcock MD 2, A Filbry PhD 4, C Arrowitz 1, F Rippke MD 4 1 Beiersdorf Inc., Wilton CT, USA 2 Mountaintop Dermatology, Colorado Springs CO, USA 3 Colorado Springs Dermatology
TM Weber PhD 1, F Samarin MD 3, M Babcock MD 2, A Filbry PhD 4, C Arrowitz 1, F Rippke MD 4 1 Beiersdorf Inc., Wilton CT, USA 2 Mountaintop Dermatology, Colorado Springs CO, USA 3 Colorado Springs Dermatology
(12) United States Patent (10) Patent No.: US 8,770,209 B2
 US008770209B2 (12) United States Patent (10) Patent No.: Kim et al. (45) Date of Patent: Jul. 8, 2014 (54) COLOR HIGHILIGHTING COSMETICS (52) U.S. Cl. CONTAINER INCLUDING ADETACHABLE USPC... 132/297: 132/318;
US008770209B2 (12) United States Patent (10) Patent No.: Kim et al. (45) Date of Patent: Jul. 8, 2014 (54) COLOR HIGHILIGHTING COSMETICS (52) U.S. Cl. CONTAINER INCLUDING ADETACHABLE USPC... 132/297: 132/318;
Identification and quantification of preservative chemicals in common household products. Session 1
 Background Session 1 Preservatives are chemicals that are commonly added to food or general such as toiletries and pharmaceuticals in order to increase their shelf lives. Preservatives can act as antimicrobials,
Background Session 1 Preservatives are chemicals that are commonly added to food or general such as toiletries and pharmaceuticals in order to increase their shelf lives. Preservatives can act as antimicrobials,
(12) United States Patent
 US009332995 B2 (12) United States Patent Russ0 et al. (54) BONE-HARVESTING TOOL (71) Applicants: Scott S. Russo, Grand Rapids, MI (US); Jeremy S. Russo, Grand Rapids, MI (US) (72) Inventors: Scott S. Russo,
US009332995 B2 (12) United States Patent Russ0 et al. (54) BONE-HARVESTING TOOL (71) Applicants: Scott S. Russo, Grand Rapids, MI (US); Jeremy S. Russo, Grand Rapids, MI (US) (72) Inventors: Scott S. Russo,
United States Patent (19)
 United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
( 12 ) United States Patent
 THAI MATA A MAI MARE MAI MULHOULUT TOUR US009795208B1 ( 12 ) United States Patent Toder ( 10 ) Patent No. : ( 45 ) Date of Patent : US 9, 795, 208 B1 Oct. 24, 2017 ( 54 ) SYSTEM AND METHOD FOR CREATING
THAI MATA A MAI MARE MAI MULHOULUT TOUR US009795208B1 ( 12 ) United States Patent Toder ( 10 ) Patent No. : ( 45 ) Date of Patent : US 9, 795, 208 B1 Oct. 24, 2017 ( 54 ) SYSTEM AND METHOD FOR CREATING
DIFFERIN DIFFERIN XP
 PRODUCT MONOGRAPH Pr DIFFERIN adapalene topical cream 0.1% w/w adapalene topical gel 0.1% w/w adapalene topical lotion 0.1% w/w Pr DIFFERIN XP adapalene topical gel 0.3% w/w Acne Therapy GALDERMA CANADA
PRODUCT MONOGRAPH Pr DIFFERIN adapalene topical cream 0.1% w/w adapalene topical gel 0.1% w/w adapalene topical lotion 0.1% w/w Pr DIFFERIN XP adapalene topical gel 0.3% w/w Acne Therapy GALDERMA CANADA
(12) United States Patent (10) Patent No.: US 6,955,816 B2
 USOO69816B2 (12) United States Patent (10) Patent No.: Klysz () Date of Patent: Oct. 18, 2005 (54) ANTI-AGING SKIN CARE COMPOSITION 2002/00638 A1 5/2002 Chung et al.... 424/1 AND USES THEREOF 2002/0106339
USOO69816B2 (12) United States Patent (10) Patent No.: Klysz () Date of Patent: Oct. 18, 2005 (54) ANTI-AGING SKIN CARE COMPOSITION 2002/00638 A1 5/2002 Chung et al.... 424/1 AND USES THEREOF 2002/0106339
Technology. HydroSal. Formulated for suspension in. water and hydro-alcoholic. environments, HydroSal. provides long lasting effects and
 Technology Technology specialized for the cosmeceutical and pharmaceutical industries. Solutions for the household, industrial, food and beverage industries. Partner with us to create custom encapsulations
Technology Technology specialized for the cosmeceutical and pharmaceutical industries. Solutions for the household, industrial, food and beverage industries. Partner with us to create custom encapsulations
POLYTAR Plus Liquid PRODUCT INFORMATION. Polytar Plus Liquid medicated scalp cleanser, contains coal tar solution.
 NAME OF THE MEDICINE POLYTAR Plus Liquid PRODUCT INFORMATION Polytar Plus Liquid medicated scalp cleanser, contains coal tar solution. DESCRIPTION Polytar Plus Liquid contains coal tar solution 4% w/w.
NAME OF THE MEDICINE POLYTAR Plus Liquid PRODUCT INFORMATION Polytar Plus Liquid medicated scalp cleanser, contains coal tar solution. DESCRIPTION Polytar Plus Liquid contains coal tar solution 4% w/w.
DIFFERIN DIFFERIN XP
 PRODUCT MONOGRAPH Pr DIFFERIN adapalene topical cream 0.1% w/w adapalene topical gel 0.1% w/w adapalene topical lotion 0.1% w/w Pr DIFFERIN XP adapalene topical gel 0.3% w/w Acne Therapy GALDERMA CANADA
PRODUCT MONOGRAPH Pr DIFFERIN adapalene topical cream 0.1% w/w adapalene topical gel 0.1% w/w adapalene topical lotion 0.1% w/w Pr DIFFERIN XP adapalene topical gel 0.3% w/w Acne Therapy GALDERMA CANADA
United States Patent (19) Frankel
 United States Patent (19) Frankel 11 Patent Number: 45 Date of Patent: Jan. 27, 1987 (54) SWEAT COLLECTING HEADBAND 76) Inventor: Alfred R. Frankel, 403 Gulf Way - Apt. 701, St. Petersburg, Fla. 33706
United States Patent (19) Frankel 11 Patent Number: 45 Date of Patent: Jan. 27, 1987 (54) SWEAT COLLECTING HEADBAND 76) Inventor: Alfred R. Frankel, 403 Gulf Way - Apt. 701, St. Petersburg, Fla. 33706
PERSONAL CARE PRODUCT OVERVIEW
 PERSONAL CARE PRODUCT OVERVIEW Personal Care Product Overview WeylCare Allantoin The ultimate cell proliferation ingredient Ointments made out of allantoin-rich comfrey roots have been used as wound healing
PERSONAL CARE PRODUCT OVERVIEW Personal Care Product Overview WeylCare Allantoin The ultimate cell proliferation ingredient Ointments made out of allantoin-rich comfrey roots have been used as wound healing
Tracey C. Vlahovic, DPM FFPM RCPS (Glasg) Clinical Professor, Dept of Podiatric Medicine, Temple Univ School of Podiatric Medicine, Philadelphia, PA
 Tracey C. Vlahovic, DPM FFPM RCPS (Glasg) Clinical Professor, Dept of Podiatric Medicine, Temple Univ School of Podiatric Medicine, Philadelphia, PA None for this presentation Ortho Dermatologics, Bako
Tracey C. Vlahovic, DPM FFPM RCPS (Glasg) Clinical Professor, Dept of Podiatric Medicine, Temple Univ School of Podiatric Medicine, Philadelphia, PA None for this presentation Ortho Dermatologics, Bako
(12) United States Patent
 (12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
(12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
 US 20100101003A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0101003 A1 McCullough (43) Pub. Date: Apr. 29, 2010 (54) 3 D HELMET WITH CROWN (22) Filed: Oct. 25, 2008 (76)
US 20100101003A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0101003 A1 McCullough (43) Pub. Date: Apr. 29, 2010 (54) 3 D HELMET WITH CROWN (22) Filed: Oct. 25, 2008 (76)
Tolerance of a Low-Level Blue and Red Light Therapy Acne Mask in Acne Patients with Sensitive Skin
 Poster 7098 Tolerance of a Low-Level Blue and Red Light Therapy Acne Mask in Acne Patients with Sensitive Skin Dara Miller 1, Michael J. Cohen 1, Adegboyega Adenaike 1, Julie Biron 2, Michael H. Gold,
Poster 7098 Tolerance of a Low-Level Blue and Red Light Therapy Acne Mask in Acne Patients with Sensitive Skin Dara Miller 1, Michael J. Cohen 1, Adegboyega Adenaike 1, Julie Biron 2, Michael H. Gold,
Physiogel. Help your patients restore skin balance and well-being
 DAILY CARE FOR DRY, SENSITIVE AND IRRITATED SKIN Help your patients restore skin balance and well-being Hypoallergenic and non-comedogenic. No fragrances, preservatives, dyes or recognised irritants DMS
DAILY CARE FOR DRY, SENSITIVE AND IRRITATED SKIN Help your patients restore skin balance and well-being Hypoallergenic and non-comedogenic. No fragrances, preservatives, dyes or recognised irritants DMS
Topical Skin Care L O O K, F E E L A N D L I V E B E T T E R
 L O O K, F E E L A N D L I V E B E T T E R Topical Skin Care Pycnogenol in Topical Skin Care Pycnogenol is widely used in topical and oral applications for various dermatological indications. A unique
L O O K, F E E L A N D L I V E B E T T E R Topical Skin Care Pycnogenol in Topical Skin Care Pycnogenol is widely used in topical and oral applications for various dermatological indications. A unique
Package leaflet: Information for the user. Tactuo 0.1% / 2.5% gel Adapalene/Benzoyl Peroxide
 Package leaflet: Information for the user Tactuo 0.1% / 2.5% gel Adapalene/Benzoyl Peroxide Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the user Tactuo 0.1% / 2.5% gel Adapalene/Benzoyl Peroxide Read all of this leaflet carefully before you start using this medicine because it contains important information
Europaisches Patentamt European Patent Office. Publication number: Office europeen des brevets EUROPEAN PATENT APPLICATION
 J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 2016.0143424A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0143424 A1 STEPHENS et al. (43) Pub. Date: May 26, 2016 (54) WEARABLE ELASTIC BAND WITH Publication Classification
(19) United States US 2016.0143424A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0143424 A1 STEPHENS et al. (43) Pub. Date: May 26, 2016 (54) WEARABLE ELASTIC BAND WITH Publication Classification
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Duac Once Daily 10 mg/g + 50 mg/g Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of gel contains: 10 mg clindamycin as clindamycin
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Duac Once Daily 10 mg/g + 50 mg/g Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of gel contains: 10 mg clindamycin as clindamycin
(12) United States Patent (10) Patent No.: US 7,188,625 B2
 US007188625B2 (12) United States Patent (10) Patent No.: US 7,188,625 B2 Durette (45) Date of Patent: Mar. 13, 2007 (54) OCULAR SURGICAL PROTECTIVE SHIELD 4,024.405 A * 5/1977 Szot... 250,516.1 5,390,373
US007188625B2 (12) United States Patent (10) Patent No.: US 7,188,625 B2 Durette (45) Date of Patent: Mar. 13, 2007 (54) OCULAR SURGICAL PROTECTIVE SHIELD 4,024.405 A * 5/1977 Szot... 250,516.1 5,390,373
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States US 20130244977A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0244977 A1 Lee et al. (43) Pub. Date: (54) COSMETIC BIO-CELLULOSE MASK PACK (30) Foreign Application Priority
(19) United States US 20130244977A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0244977 A1 Lee et al. (43) Pub. Date: (54) COSMETIC BIO-CELLULOSE MASK PACK (30) Foreign Application Priority
[ ] Reduces Swelling. Improves Skin. Fred Attention Night Solution Fred Chewable Tab & Fred Cream. Project The Newer Skin.
![[ ] Reduces Swelling. Improves Skin. Fred Attention Night Solution Fred Chewable Tab & Fred Cream. Project The Newer Skin. [ ] Reduces Swelling. Improves Skin. Fred Attention Night Solution Fred Chewable Tab & Fred Cream. Project The Newer Skin.](/thumbs/78/78772438.jpg) Fred Attention Night Solution Fred Chewable Tab & Fred Cream Reduces Swelling Project The Newer Skin Improves Skin [3 months supply] Chew & Apply For intake and skin application In&Out Collaboration Baby
Fred Attention Night Solution Fred Chewable Tab & Fred Cream Reduces Swelling Project The Newer Skin Improves Skin [3 months supply] Chew & Apply For intake and skin application In&Out Collaboration Baby
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O18O194A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180194 A1 Cutter (43) Pub. Date: Jul.19, 2012 (54) GARMENTS WITH ADJUSTABLE WAISTS (52) U.S. Cl.... 2/237
(19) United States US 2012O18O194A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180194 A1 Cutter (43) Pub. Date: Jul.19, 2012 (54) GARMENTS WITH ADJUSTABLE WAISTS (52) U.S. Cl.... 2/237
Angel Yeast Cosmetic Ingredients
 Angel Yeast Cosmetic Ingredients Carboxymethyl yeast beta-glucan(cmg) C90... 2 Yeast Extract E100... 5 Yeast Nucleotides N80... 8 Zinc-enriched Yeast Extract Z20...11-1 - Carboxymethyl yeast beta-glucan(cmg)
Angel Yeast Cosmetic Ingredients Carboxymethyl yeast beta-glucan(cmg) C90... 2 Yeast Extract E100... 5 Yeast Nucleotides N80... 8 Zinc-enriched Yeast Extract Z20...11-1 - Carboxymethyl yeast beta-glucan(cmg)
USOO A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999
 USOO5996780A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999 54 COSMETIC APPARATUS 3.513,830 5/1970 Kalayjian... 128/2 3,640,268 2/1972 Davis...... 128/2
USOO5996780A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999 54 COSMETIC APPARATUS 3.513,830 5/1970 Kalayjian... 128/2 3,640,268 2/1972 Davis...... 128/2
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 TOPICAL CLINDAMYCIN PRODUCTS: ACANYA (clindamycin phosphate-benzoyl peroxide) gel BENZACLIN (clindamycin phosphate-benzoyl peroxide) gel CLEOCIN-T (clindamycin phosphate) gel, lotion, solution, swab CLINDAGEL
TOPICAL CLINDAMYCIN PRODUCTS: ACANYA (clindamycin phosphate-benzoyl peroxide) gel BENZACLIN (clindamycin phosphate-benzoyl peroxide) gel CLEOCIN-T (clindamycin phosphate) gel, lotion, solution, swab CLINDAGEL
CREAMS BATH SOAP SUBSTITUTES OINTMENT GEL ADDITIVES. NEW 500g. pump
 CREAMS BATH ADDITIVES SOAP SUBSTITUTES OINTMENT GEL NEW NEW 500g pump The Zeroderma range of emollients offers similar products to leading brands with no compromise on patient care and cost s of up to
CREAMS BATH ADDITIVES SOAP SUBSTITUTES OINTMENT GEL NEW NEW 500g pump The Zeroderma range of emollients offers similar products to leading brands with no compromise on patient care and cost s of up to
Intravenous Access and Injections Through Tattoos: Safety and Guidelines
 CADTH RAPID RESPONSE REPORT: SUMMARY OF ABSTRACTS Intravenous Access and Injections Through Tattoos: Safety and Guidelines Service Line: Rapid Response Service Version: 1.0 Publication Date: August 03,
CADTH RAPID RESPONSE REPORT: SUMMARY OF ABSTRACTS Intravenous Access and Injections Through Tattoos: Safety and Guidelines Service Line: Rapid Response Service Version: 1.0 Publication Date: August 03,
