(12) United States Patent
|
|
|
- Lynne Hardy
- 6 years ago
- Views:
Transcription
1 (12) United States Patent US B1 (10) Patent N0.: Sefton (45) Date of Patent: *Jul. 17, 2001 (54) METHOD AND COMPOSITION FOR 5,516,950 5/1996 Piccariello et al.. TREATING ACNE 5,538,732 * 7/1996 Smith /402 5,543,417 * 8/1996 Waldstreicher /284 (75) Inventor: John Sefton, Trabuco Canyon, CA (US) glemardozl it al' - arren e a..,, (73) Assignee: Allergan Sales, Inc., Irvine, CA (US) 13:0211; a1 (*) Notice: This patent issued on a continued pros * grlgtgaeitail' ' 424/401 ecution application?led under 37 CFR 5:665:364 * 9/1997 McAtee et al / (d), and is subject to the twenty year patent term provisions of 35 U.S.C. FOREIGN PATENT DOCUMENTS 4(a)(2)' * 3/1999 (EP). Subject to any disclaimer, the term of this OTHER PUBLICATIONS atent is extended or ad'usted under 35 $130 4(k)) by 0 days] Leyden et al. Evaluation of the Evaluation of the antimicro bial effects in vivo of TriaZ..., J. of Dermatological (21) _ Appl' NO" 09/ Treatment, 1997, vol. 8/2, pp. S7 S10.* Tucker et al. Comparison of Topical Clindamycin (22) Filed: Feb_ PhOS..., BR. J. Dermatol., 1984, VOl. 110/4, pp: * Gloor et al. Topical treatment of acne vulgaris..., Z. (51) Int. Cl A61K 31/20; A61K 31/075 Hautkr., 1982 vol. 57/12, pp , 875, 878* (52) US. Cl /558; 514/714 stogmflnn W7 Recommendations_ for treatment of acne (58) Field Of Search /198, 284, klgagsgs P , 1993, vol-28b, pp _ ' / Gollnick et al., Topical therapy in acne, Journal of the - Euro ean Academ of Dermatolo and Venereolo, vol. (56) References Cited ll/sulgp. pp (S8_Sy12), 1998~$ gy gy U.S. PATENT DOCUMENTS Mackrides et al., AZelaic acid therapy for acne, American _ Family Physician, vol. 54/8, pp , 1996* /1974 Blrkenmeyer' AZelex(aZelaic acid cream 20%) package insert, Allergan 3,932,653 1/1976 Stoughton. herbert 1995* itqucgl?toai h Gollnick, et al., Topical drug treatment in acne, Dermatolo /1976 ngj'gdnznkzng~ gy(base1)> VOL 196(1) 1" *.. 4 O /1977 A er et a1 ' Van Hoogdalem, Transdermal absorption of topical /1979 sggughton' _' anti acne..., J. of the European Academy of Derm. and 4,323,558 >x< 4/1982 Nelson Kligman. et a1~ ' Venen,,. - V ,386,104 5/1983 NaZZaro-Porro. cued by exammer 4,387,107 6/1983 Klein 6t a1~ - Primary Examiner William R. A. Jarvis /1983 Roques et al' - Assistant Examiner Vickie Kim gg?llzfgnet a1" (74) Attorney, Agent, or Firm Cynthia H. O Donohue; 4:6O7:1O1 8/1986 Bernstein ' Robert J. Baran; Martm A. Voet 4,621,075 11/1986 FaWZi et al.. (57) ABSTRACT 4,671,956 6/1987 Bouillon et al.. 4,692,329 9/1987 K199} et a1~ - The present invention provides a method for treating acne 4,743,588 5/1988 Mlrelovsky et a1- - vulgaris by serially applying a topical composition of azelaic /1988 Maklno et al' - acid and a topical composition of benzoyl peroxide. The /1988 Makmo et a1 ' present invention also provides topical compositions of a 4,803, /1989 Jacquet 61 al.. A989 Nakagawa et a1 f b 1 d 1 ' perox1 e o enzoy perox1 e, an ' aze a1c ac1 d t an 1 s 4 9O /199O J t t 1 ' ' derivatives, such as azela1c acid, sodium salt or methylester M1990 Fiflillreeteala ' ' Which are useful for treating acne vulgaris and may be used 4:942:031 7/1990 Levin _ ' ' to simultaneously apply benzoyl peroxide and azelaic acid. 5,231,087 7/1993 Thornfeldt. 5,260,292 * 11/1993 Robinson et al / Claims, N0 Drawings
2 1 METHOD AND COMPOSITION FOR TREATING ACNE FIELD OF THE INVENTION This invention relates to a method and composition for treating acne vulgaris. BACKGROUND OF THE ART Acne vulgaris is an in?ammatory disease of the sebaceous glands characterized by an eruption of the skin, often pustular in nature but not suppurative. Acne is a common af?iction of the adolescent and affects a small but signi?cant percentage of the adult population. Acne involvement results in unsightly lesions, particularly on the face, and in some cases results in severe scarring. Various topical agents are utilized in the treatment of acne and these include sulfur, resorcinol, salicylic acid, benzoyl peroxide, retinoids and topical antibiotics. An effective anti-acne agent (or composition) must exhibit the following activities: (a) a sebostatic activity so as to inhibit hyperseborrhea; (b) a keratolytic and comedolytic activity so as to avoid hyperkeratosis of the follices and to permit removal of comedos; (c) a bacteriostatic activity so as to inhibit the activity of Propionibacterium acnes. Nevertheless, acne vulgaris is seldom cured and only can be contained With dif?culty. The antibiotic clindamycin has been used, topically, to treat acne vulgaris. (See US. Pat. No. 3,969,516, to Stoughton.) Various references discuss the use of vehicle formulations to enhance the ef?cacy of topically-applied clindamycin. (See US. Pat. Nos. 3,932,653; 3,989,816; 3,991,203; 4,132,781; 4,671,956; 4,746,675; 4,789,667; 4,803,228 and 4,882,359.) Clindamycin salts, clindamycin derivatives, and various dosage forms of clindamycin have also been discussed as a treatment for acne vulgaris. (See US. Pat. Nos. 3,849,396; 4,621,075 and 4,916,118.) Finally, combinations of clindamycin and other compounds active for the treatment of acne vulgaris are disclosed in US. Pat. Nos. 4,323,558; 4,505,896; 4,607,101; 4,906,617; 4,942,031 and 4,018,918. BenZoyl peroxide has been suggested for treating acne vulgaris. (See US. Pat. No. 4,387,107.) For many years, benzoyl peroxide has been proven to be a particularly powerful keratolytic and anti-seborrhic agent, as Well as being endowed With antibacterial properties. Topical ben Zoyl peroxide compositions, including a vehicle to enhance the ef?cacy thereof, are known (See US. Pat. No. 4,411, 893). Topical compositions of benzoyl peroxide combina tion With antibiotics are also known. (See US. Pat. Nos. 4,407,794; 4,692,329 and 4,387,107) Peroxides, other than benzoyl peroxide, have been sug gested for treatment of acne vulgaris, alone, or in combina tion With other compounds useful in treating acne vulgaris. (See US. Pat. Nos. 4,607,101 and 4,906,617.) These per oxides are suggested as having certain advantages, e.g. stability over benzoyl peroxide. US. Pat. No. 4,671,956 identi?es the problem of benzoyl peroxide decomposing coingredients in topical formulations to thereby cause itch ing upon application. It is suggested that this problem may be solved by including a sunscreen in the topical formulation to retard this decomposition effect of benzoyl peroxide. AZelaic acid has been used topically and systemically to treat acne. See, for example, US. Pat. No. 4,386,104 to NaZZo-Pavarro. In view of the above, it is apparent that there is a great deal of interest in utilizing topical compositions for the treatment of acne vulgaris, such compositions utilizing as an active ingredient clindamycin or benzoyl peroxide or azelaic acid, alone, or in combination With other active ingredients for the treatment of acne vulgaris. Therefore, one object of the instant invention is to provide a method of treating acne vulgaris With topical compositions including benzoyl peroxide and azelaic acid. It is another object of the invention to provide a method of treatment of acne vulgaris by topical application of compositions of benzoyl peroxide in a gel form and azelaic acid in a cream form. It is another object of this invention to provide composi tions for the topical treatment of acne vulgaris. Another object of the invention is to provide topical compositions of benzoyl peroxide and azelaic acid that may be used for treating acne vulgaris. Other objects and advantages of the instant invention Will become apparent from a careful reading of the speci?cation below. SUMMARY OF THE INVENTION The present invention provides a method for treating acne vulgaris by topically applying a composition of benzoyl peroxide and azelaic acid, serially, in a therapeutically effective amount. The azelaic acid or its pharmaceutically acceptable salts or prodrugs (e.g. azelaic acid, sodium salt or lower alkyl ester), may be applied in an amount sufficient to provide from about 0.1 to about 30 percent, and preferably from about 0.5 to about 25 percent, by Weight, eg about 20 percent azelaic acid. BenZoyl peroxide, Which has kera tolytic and antiseborrheic properties, may be present in an amount suf?cient to provide from about 0.1 to about 30 percent, and preferably from about 2.5 to about 10 percent, by Weight, benzoyl peroxide. The benzoyl peroxide may more preferably be used as hydrous benzoyl peroxide and may be suspended preferably in the form of microparticles. The above described topical composition may be in the form of a solution, gel, ointment, cream, a liquid suspension or emulsion or a stick base. DETAILED DESCRIPTION OF THE INVENTION The compositions of this invention are administered topi cally to treat acne vulgaris. That is, the compositions may be applied as a solution, gel, ointment, cream, a liquid suspen sion or emulsion or a stick base. Thus, it is preferred that such compositions include a pharmaceutically acceptable carrier that enhances the ef?cacy of such topical adminis tration. Pharmaceutically acceptable carriers include con ventional emulsi?ers, such as fatty alcohols, glycol ethers and esters of fatty acids; conventional emollients, such as isopropyl and butyl esters of fatty acids, eg isopropyl myristate; humectants such as glycerin, propylene glycol, polyethylene glycol; and alcohols and acetone; oils such as mineral oil, petroleum oil, oil extracts from animal or vegetable sources; conventional stabilizers including anti oxidants and preservatives. The compositions may also include agents, such as urea, to improve the hydration of the skin. In addition to the foregoing conventional formulations, the topical compositions may include penetration-enhancing agents such as 1-pyrrolidone and N-loWer alkyl-2 pyrrolidones, such as N-methyl-2-pyrrolidone; and 1-substituted azacycloalkan-2-ones such as, for example, 1-n-dodecylaZacycloheptan-2-one and other compounds disclosed in US. Pat. No. 3,989,816. Longer chain sulfoxides, e.g., n-octyl methyl sulfoxide and hexamethylene-lauramide and the other penetration enhancing agents disclosed in US. Pat. No. 4,743,588, may also be included in the formulations utilized in the method of this invention. The amount of these penetration
3 3 enhancing agents Which may be used in the present inven tion ranges from about 0.1 to 25 percent and preferably about 1 to percent by Weight of the composition. The amount of the compositions to be administered Will obviously be an effective amount for the desired result expected therefrom. This, of course, Will be ascertained by the ordinary skill of the practitioner. In accordance With the usual prudent formulating practices, a dosage near the lower end of the useful range of the particular agent may be employed initially and the dosage increased as indicated from the observed response, as in the routine procedure of the physician. In carrying out the novel method employing the topical route, the active ingredient(s) formulated, for example, as a gel or lotion or suspension, is applied to the affected area of the skin at a rate varying from 0.2 mg per square cm of skin surface per day up to 10 mg per square cm of skin surface per day until the appearance of the affected skin has returned to normal. The gel or lotion or suspension is generally applied for several days The topical composition, including benzoyl peroxide, is preferably a gel formulation. Typically, said gel formulation may comprise: FORMULATION B 4 or 8 percent, by Weight, benzyl peroxide in a gel vehicle containing puri?ed Water, cetyl alcohol dimethyl isosorbide, fragrance, simethicone, stearyl alcohol and ceteareth-20. An example of such a product is Brevoxyl benzyl peroxide available from Stiefel Laboratories, Inc., Coral Gables, Fla. The invention is further illustrated by the following formulations and examples Which are illustrative of a spe The topical Compositions of this invention may be applied 20 ci?c mode of practicing the invention and is not intended as to the face of a patient With acne 1 to 4 times daily With the limiting the Scope of the C1aim5~ result that open and closed comedones are markedly reduced Within two to four Weeks. The topical composition, including azaleic acid, is pref- 25 EXAMPLE 1 erably a cream formulation. Typically, said cream formula tion may comprise: A 20 year old male applies 0.35 gms of the Formulation A and 0.35 gms of Formulation B to his face 4 times daily, each. After 10 days, the number of comedones begin to w 30 diminish. By the end of four Weeks, the number of come INGREDIENTS WEIGHT PERCENT dones declines signi?cantly. AZELAIC ACID BENZOIC ACID DAB 0.20 PROPYLENE GLYCOL USP EXAMPLE 2 CUTINA CBS(1) 7.00 PEG-5 GLYCERYL STEARATE(2) 5.00 CETEARYL OCTANOATEG) patients Were treated, serially, With Formulation A and GLYCERIN (85%) DAB 1.5 F 1 t. B dt t 1 29 t. t PURIFIED WATER D AB ) Q5 ormu a ion as compare 0 a con ro group 0 pa ien s 40 WhlCh Was treated With BenZamyc1n acne medication, 1. Glyceryl Stearate (and) Cetearyl Alcohol (and) Cetyl Palmitate (and) comprising benzyl peroxide 5% and erthyromycin 3%, by goicr filécnegiggégg?qgle) Weight, available from Dermik Laboratories, Inc., 3_ PCL Liquid (DRAGOCO) Collegev1lle, Pa patients from each group com percent (w/w) pleted the study. The demographic data for these two groups are reported in Table 1, below. TABLE 1 Demographic Data AZelex/BP Benzamycin Male Female Total Male Female Total Age: n 11 (37.9%) 18 (62.1%) 29 5 (17.2%) 24 (82.8%) 29 mean b 30.7b 28.9 SD min InaX Race: Black 5 (45.4%) 10 (55.6%) (51.7%) 0 (62.5%) (51.7%) Caucasian 6 (54.6%) 7 (38.9%) 13 (44.8%) 5 (100%) 9 (37.5%) 14 (48.3%) Hispanic Oriental 0 1 (5.6%) 1 (3.4%) Other Acne <1 year HX:d 1-2 years 6 (54.6%) 1 (5.6%) 7 (24.1%) 2 (40.0%) 0 2 (6.9%) 3-5 years 1 (9.1%) 3 (16.7%) 4 (13.8%) 2 (40.0%) 2 (8.3%) 4 (13.8%)
4 5 TABLE l-continued Demographic Data AZeleX BP Benzamvcin Male Female Total Male Female Total 6-10 years 1 (9.1%) 5 (27.8%) 6 (20.7%) 1 (20.0%) 9 (37.5%) 10 (34.5%) >10 years 3 (27.3%) 9 (50.0%) 12 (41.4%) (%) 13 (54.2%) 13 (44.8%) 3p < 0.05, within AZeleX/BP group, for age between, male female bp < 0.05, within Benzamycin group, for age between, male female Cp < 0.05, within Benzamycin group, for race between, male female dp < 0.05, within Benzamycin group, for acne history between, male female The patients were?rst evaluated for the severity of their acne conditions. This evaluation is reported in Table 2, below. TABLE 2 Overall Disease Severity Change from Baseline Visit Treatment Week 0 Number Group n mean SD p-value n mean SD p-value 1 (Week 0) AzeleX/BP Benzamycin (Week 4) AzeleX/BP Benzamycin (Week 8) AzeleX/BP Benzamycin (Week 12) AzeleX/BP Benzamycin Note: Change from baseline = B.L. Visit value minus follow-up Visit value (positive value indi cates a decrease from baseline) Scale (Overall Evaluation of Disease Severity): 0 = None Normal 1 = Condition is present, but is less than mild 2 = Mild Condition is slightly noticeable 3 = Condition is worse than mild, but less than moderate 4 = Moderate Condition is noticeable 5 = Condition is worse than moderate, but less than severe 6 = Severe Condition is very distinctive Over the course of twelve weeks of treatment, the lesion 45 count-pustules and papules are evaluated to determine the effect of the treatment. See Tables 3 and 4. TABLE 3 Overall Disease Severity 50 Improvement From Baseline w N 55 v1 >.0 60 Week I AzeleX/BPO 131E: Benzamycin 65
5 7 TABLE 4 Lesion Count Pustules Change from Baseline Visit Treatment Week 0 Number Group n mean SD p-value n mean SD p-value 1 (Week 0) Azelex/BP Benzamycin (Week 4) Azelex/BP Benzamycin (Week 8) Azelex/BP Benzamycin (Week 12) Azelex/BP Benzamycin As reported in Tables 3 and 4, above, the serial treatment With azaleic acid and benzoyl peroxide is signi?cantly more effective for treating acne than the BenZamycin control. Similar evaluations for open comedones, closed come dones and in?ammatory lesions show that the method of this invention is more effective than the control. As to the side effects of treating patients for acne by the method of the invention and the control, the following results Were obtained. Scaling. Erythema. Dryness. Oilness. Burning. Itching. Azaleic acid (AA)/Benzylperoxide (BPO) is slightly better than the control. AA/BPO is better than the contol. AA/BPO is equal to or slightly Worse than the control. While particular embodiments of the invention have been described, it Will be understood, of course, that the invention is not limited thereto since many obvious modi?cations can be made; and it is intended to include Within this invention any such modi?cations as Will fall Within the scope of the appended claims. For example, it Will be appreciated by those skilled in the art that various pharmaceutically accept able derivatives, salts and prodrugs of azelaic acid, eg azelaic acid, sodium or potassium salt, or lower alkyl ester, i.e. C1 to C6 alkyl ester, e.g. methyl azelate, may be used in place of azaleic acid. Also, various forms of peroxides may be used in place of hydrous benzoyl peroxide; i.e., diaryl peroxide, alkyl aryl peroxide, cycloalkyl aryl peroxide, may be substituted for hydrous benzoyl peroxide. For example, lauroyl benzoyl peroxide, cyclohexyl carbanolyl benzoyl peroxide may be used in place of benzoyl peroxide. Also, the azaleic acid and benzyl peroxide may be com bined in a single topical composition, e.g., a cream or gel, for ease of application Typically, said single topical composition Will comprise the amounts of azaleic acid and benzoyl peroxide suf?cient to provide the amounts described above for serial applica tion in a single topical application. What is claimed is: 1. A topical composition for treating acne vulgaris in human patients consisting essentially of a therapeutically effective amount of benzoyl peroxide and azelaic acid in the absence of retinoids or 5-alpha reductase inhibitors. 2. The composition of claim 1 Wherein said benzoyl peroxide and azelaic acid are dispersed in a pharmaceuti cally acceptable carrier. 3. The composition of claim 2 Wherein said composition is a cream or gel. 4. The composition of claim 3 Wherein said composition comprises from about 0.1 to 30 percent, by Weight, azelaic acid. 5. The composition of claim 4 Wherein said composition comprises from about 2.5 to 10 percent, by Weight, benzoyl peroxide. 6. The composition of claim 5 Wherein said composition comprises about 0.5 to 25 percent, by Weight, azelaic acid. 7. The composition of claim 6 Wherein said composition comprises about 20 percent, by Weight, azelaic acid. 8. A method of treating acne vulgaris in human patients composition according to claim A method of treating acne vulgaris in human patients composition according to claim A method of treating acne vulgaris in human patients composition according to claim 7.
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0165220 A1 Chang et al. US 201701 65220A1 (43) Pub. Date: (54) (71) (72) (21) (22) (60) (60) TOPCAL PHARMACEUTICAL FORMULATIONS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0165220 A1 Chang et al. US 201701 65220A1 (43) Pub. Date: (54) (71) (72) (21) (22) (60) (60) TOPCAL PHARMACEUTICAL FORMULATIONS
12) United States Patent 10) Patent No.: US 6,433,024 B1
 USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
(12) United States Patent (10) Patent No.: US 6,841,523 B1
 USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
The solution contains isopropyl alcohol 50% v/v, propylene glycol, and water.
 Cleocin T (clindamycin phosphate topical solution, USP) (clindamycin phosphate topical gel) (clindamycin phosphate topical lotion) For External Use DESCRIPTION CLEOCIN T Topical Solution and CLEOCIN T
Cleocin T (clindamycin phosphate topical solution, USP) (clindamycin phosphate topical gel) (clindamycin phosphate topical lotion) For External Use DESCRIPTION CLEOCIN T Topical Solution and CLEOCIN T
(12) United States Patent (10) Patent No.: US 6,752,627 B2
 USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
United States Patent (19)
 USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
(12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001
 US006257248B1 (12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001 (54) BOTH HAND HAIR CUTTING METHOD 5,991,918 * 11/1999 Choate..... 2/21 6,079,107 * 6/2000
US006257248B1 (12) United States Patent (10) Patent N0.: US 6,257,248 B1 Yeh (45) Date of Patent: Jul. 10, 2001 (54) BOTH HAND HAIR CUTTING METHOD 5,991,918 * 11/1999 Choate..... 2/21 6,079,107 * 6/2000
(12) United States Patent
 US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
United States Patent (19) Humbrecht
 United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
United States Patent (19)
 United States Patent (19) Young 54) THERAPEUTIC COMPOSITION (75) Inventor: Henry Y. Young, Delmar, N.Y. 73) Assignee: Stiefel Laboratories, Inc., Oak Hill, N.Y. (21) Appl. No.: 1,773. 22). Filed: Apr.
United States Patent (19) Young 54) THERAPEUTIC COMPOSITION (75) Inventor: Henry Y. Young, Delmar, N.Y. 73) Assignee: Stiefel Laboratories, Inc., Oak Hill, N.Y. (21) Appl. No.: 1,773. 22). Filed: Apr.
(12) United States Patent (10) Patent No.: US 6,308,717 B1
 USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
United States Patent (19) Katz
 United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
Children s Hospital Of Wisconsin
 Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BREVOXYL HYDROPHASE. Benzoyl Peroxide Cream IP
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BREVOXYL HYDROPHASE Benzoyl Peroxide Cream IP QUALITATIVE AND QUANTITATIVE COMPOSITION Hydrous Benzoyl Peroxide IP equivalent
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BREVOXYL HYDROPHASE Benzoyl Peroxide Cream IP QUALITATIVE AND QUANTITATIVE COMPOSITION Hydrous Benzoyl Peroxide IP equivalent
(12) United States Patent (10) Patent No.: US 6,422,036 B1. Giannis et al. (45) Date of Patent: Jul. 23, 2002
 USOO6422036B1 (12) United States Patent (10) Patent No.: US 6,422,036 B1 Giannis et al. (45) Date of Patent: Jul. 23, 2002 (54) JEWELRY CLASP 4,611,368 9/1986 Battersby... 24/116 R 5,214,940 A * 6/1993
USOO6422036B1 (12) United States Patent (10) Patent No.: US 6,422,036 B1 Giannis et al. (45) Date of Patent: Jul. 23, 2002 (54) JEWELRY CLASP 4,611,368 9/1986 Battersby... 24/116 R 5,214,940 A * 6/1993
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BREVOXYL CREAMY WASH. Benzoyl Peroxide Creamy Wash 4%
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BREVOXYL CREAMY WASH Benzoyl Peroxide Creamy Wash 4% QUALITATIVE AND QUANTITATIVE COMPOSITION Hydrous Benzoyl Peroxide
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BREVOXYL CREAMY WASH Benzoyl Peroxide Creamy Wash 4% QUALITATIVE AND QUANTITATIVE COMPOSITION Hydrous Benzoyl Peroxide
III. United States Patent 19 Jordan 5,389,129. Feb. 14, ). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee:
 United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
(19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
Item/Package Details Size Item Bottle ph Shelf Life 1.0 oz/29.6 ml 1101 Lucite Matte Silver Pump months
 B-Kleer 10 Lotion Use of mild to moderate acne. Gently exfoliates encrustation and acne pustules. Penetrates pores to eliminate most acne pimples. Reduces the severity of acne blemishes. Help prevent new
B-Kleer 10 Lotion Use of mild to moderate acne. Gently exfoliates encrustation and acne pustules. Penetrates pores to eliminate most acne pimples. Reduces the severity of acne blemishes. Help prevent new
Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts this compound to the
 APPROVED PACKAGE INSERT SCHEDULING STATUS:S4 PROPRIETARY NAME AND DOSAGE FORM: DALACIN T ( Solution) DALACIN T (Lotion) COMPOSITION: DALACIN T Solution contains the following per ml : Clindamycin phosphate
APPROVED PACKAGE INSERT SCHEDULING STATUS:S4 PROPRIETARY NAME AND DOSAGE FORM: DALACIN T ( Solution) DALACIN T (Lotion) COMPOSITION: DALACIN T Solution contains the following per ml : Clindamycin phosphate
THE SCIENCE WHITE PAPER SERIES OF IMAGE SKINCARE: by Marc A. Ronert MD PhD, Clinical Director Image Skincare
 THE SCIENCE WHITE PAPER SERIES OF IMAGE SKINCARE: Clear Cell Line containing Benzoyl Peroxide by Marc A. Ronert MD PhD, Clinical Director Image Skincare ABSTRACT Image Skincare offers products with many
THE SCIENCE WHITE PAPER SERIES OF IMAGE SKINCARE: Clear Cell Line containing Benzoyl Peroxide by Marc A. Ronert MD PhD, Clinical Director Image Skincare ABSTRACT Image Skincare offers products with many
(12) United States Patent
 USOO8663699B2 (12) United States Patent Chang et al. (54) TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING ALOW CONCENTRATION OF BENZOYL PEROXDE IN SUSPENSION IN WATER ANDA WATER-MSCIBLE ORGANIC SOLVENT
USOO8663699B2 (12) United States Patent Chang et al. (54) TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING ALOW CONCENTRATION OF BENZOYL PEROXDE IN SUSPENSION IN WATER ANDA WATER-MSCIBLE ORGANIC SOLVENT
JDDonline.com. Introduction. Methods Study Design Twenty-three patients enrolled in a 4-week, multicenter, investigator-blinded,
 534 Emil Tanghetti MD, a Leon Kircik MD, b David Wilson MD, c Sunil Dhawan MD d a. Center for Dermatology and Laser Surgery, Sacramento, CA b. Physicians Skin Care PLLC, Louisville, KY c. Education and
534 Emil Tanghetti MD, a Leon Kircik MD, b David Wilson MD, c Sunil Dhawan MD d a. Center for Dermatology and Laser Surgery, Sacramento, CA b. Physicians Skin Care PLLC, Louisville, KY c. Education and
Ref. 11. (12) Patent Application Publication (10) Pub. No.: US 2012/ A1. (19) United States. Polstein et al. (43) Pub. Date: Jun.
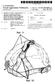 (19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
EFFECTIVE PRIMARY CARE MANAGEMENT OF ACNE VULGARIS
 EFFECTIVE PRIMARY CARE MANAGEMENT OF ACNE VULGARIS WHY ACNE? EXCESS OIL PRODUCTION BY OVERACTIVE SEBACEOUS GLANDS. IMPROPER CELL TURNOVER/EXFOLIATION CLOGS PORES. DISRUPTED OXYGEN SUPPLY ALLOWS P. ACNE
EFFECTIVE PRIMARY CARE MANAGEMENT OF ACNE VULGARIS WHY ACNE? EXCESS OIL PRODUCTION BY OVERACTIVE SEBACEOUS GLANDS. IMPROPER CELL TURNOVER/EXFOLIATION CLOGS PORES. DISRUPTED OXYGEN SUPPLY ALLOWS P. ACNE
(12) United States Patent (10) Patent No.: US 7434,929 B2
 US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
(12) United States Patent
 (12) United States Patent USOO7374282B2 (10) Patent No.: US 7,374.282 B2 Tendler (45) Date of Patent: May 20, 2008 (54) METHOD AND APPARATUS FOR VIEWING 6,623,116 B2 * 9/2003 Kerns et al.... 351,165 POLARIZED
(12) United States Patent USOO7374282B2 (10) Patent No.: US 7,374.282 B2 Tendler (45) Date of Patent: May 20, 2008 (54) METHOD AND APPARATUS FOR VIEWING 6,623,116 B2 * 9/2003 Kerns et al.... 351,165 POLARIZED
(12) United States Patent (10) Patent No.: US 6,582,709 B1
 USOO82709B1 (12) United States Patent (10) Patent No.: Maor et al. () Date of Patent: Jun. 24, 2003 (54) CREAM COMPOSITION COMPRISING (56) References Cited DEAD SEA MUD U.S. PATENT DOCUMENTS (75) Inventors:
USOO82709B1 (12) United States Patent (10) Patent No.: Maor et al. () Date of Patent: Jun. 24, 2003 (54) CREAM COMPOSITION COMPRISING (56) References Cited DEAD SEA MUD U.S. PATENT DOCUMENTS (75) Inventors:
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
(19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
CREAMS BATH SOAP SUBSTITUTES OINTMENT GEL ADDITIVES. NEW 500g. pump
 CREAMS BATH ADDITIVES SOAP SUBSTITUTES OINTMENT GEL NEW NEW 500g pump The Zeroderma range of emollients offers similar products to leading brands with no compromise on patient care and cost s of up to
CREAMS BATH ADDITIVES SOAP SUBSTITUTES OINTMENT GEL NEW NEW 500g pump The Zeroderma range of emollients offers similar products to leading brands with no compromise on patient care and cost s of up to
(12) United States Patent (10) Patent No.: US 6,413,305 B1
 USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
(12) (10) Patent No.: US 6,971,424 B1. Angevine (45) Date of Patent: Dec. 6, (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi...
 United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0231267 A1 Mendoza et al. US 20150231267A1 (43) Pub. Date: (54) (71) (72) (21) (22) (86) METHOD FOR PRODUCING EXTENDED-RELEASE
INCI Name: Cyclopentasiloxane (and) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (and) PEG/PPG-20/23 Dimethicone
 Technical Data Sheet Velvesil Plus Velvesil* Plus Gel INCI Name: Cyclopentasiloxane (and) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (and) PEG/PPG-20/23 Dimethicone Product Description Velvesil Plus
Technical Data Sheet Velvesil Plus Velvesil* Plus Gel INCI Name: Cyclopentasiloxane (and) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (and) PEG/PPG-20/23 Dimethicone Product Description Velvesil Plus
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 TOPICAL CLINDAMYCIN PRODUCTS: ACANYA (clindamycin phosphate-benzoyl peroxide) gel BENZACLIN (clindamycin phosphate-benzoyl peroxide) gel CLEOCIN-T (clindamycin phosphate) gel, lotion, solution, swab CLINDAGEL
TOPICAL CLINDAMYCIN PRODUCTS: ACANYA (clindamycin phosphate-benzoyl peroxide) gel BENZACLIN (clindamycin phosphate-benzoyl peroxide) gel CLEOCIN-T (clindamycin phosphate) gel, lotion, solution, swab CLINDAGEL
COST-EFFECTIVE ACNE MANAGEMENT PEGGY VERNON, RN, MA, C-PNP, DCNP, FAANP
 COST-EFFECTIVE ACNE MANAGEMENT PEGGY VERNON, RN, MA, C-PNP, DCNP, FAANP Disclosures There are no financial relationships with commercial interests to disclose Ay unlabeled/unapproved uses of drugs or products
COST-EFFECTIVE ACNE MANAGEMENT PEGGY VERNON, RN, MA, C-PNP, DCNP, FAANP Disclosures There are no financial relationships with commercial interests to disclose Ay unlabeled/unapproved uses of drugs or products
Europaisches Patentamt European Patent Office. Publication number: Office europeen des brevets EUROPEAN PATENT APPLICATION
 J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
J Europaisches Patentamt European Patent Office Publication number: 0 244 859 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87106617.1 Int. Cl.4:A61K 7/48 Date of filing:
Management of acne requires proper application
 DRUG THERAPY TOPICS A Qualitative and Quantitative Assessment of the Application and Use of Topical Acne Medication by Patients James Q. Del Rosso, DO Management of acne requires proper application of
DRUG THERAPY TOPICS A Qualitative and Quantitative Assessment of the Application and Use of Topical Acne Medication by Patients James Q. Del Rosso, DO Management of acne requires proper application of
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 TOPICAL RETINOID AND COMBINATION PRODUCTS: ATRALIN (tretinoin) gel AVITA (tretinoin) cream and gel DIFFERIN (adapalene) cream, gel, lotion (Over-the-Counter Differin is a plan exclusion) EPIDUO (adapalene-benzoyl
TOPICAL RETINOID AND COMBINATION PRODUCTS: ATRALIN (tretinoin) gel AVITA (tretinoin) cream and gel DIFFERIN (adapalene) cream, gel, lotion (Over-the-Counter Differin is a plan exclusion) EPIDUO (adapalene-benzoyl
United States Patent (19)
 United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
New Zealand Datasheet
 New Zealand Datasheet 1 PRODUCT NAME Epiduo 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% / benzoyl peroxide 2.5% gel 3 PHARMACEUTICAL FORM Epiduo is a white to very pale yellow opaque gel
New Zealand Datasheet 1 PRODUCT NAME Epiduo 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Adapalene 0.1% / benzoyl peroxide 2.5% gel 3 PHARMACEUTICAL FORM Epiduo is a white to very pale yellow opaque gel
USOO A United States Patent (19) 11 Patent Number: 6,117,843 Baroody et al. (45) Date of Patent: *Sep. 12, 2000
 USOO611.7843A United States Patent (19) 11 Patent Number: Baroody et al. () Date of Patent: *Sep. 12, 2000 54) COMPOSITIONS FOR THE TREATMENT OF No. 08/2,1, Apr. 28, 1994, Pat. No. 5,733,886, which is
USOO611.7843A United States Patent (19) 11 Patent Number: Baroody et al. () Date of Patent: *Sep. 12, 2000 54) COMPOSITIONS FOR THE TREATMENT OF No. 08/2,1, Apr. 28, 1994, Pat. No. 5,733,886, which is
Moisturizing Lotion with Laurest 1220 and Squalane
 Moisturizing Lotion with Laurest 220 and Squalane In this formulation, Laurest 220 acts as an emollient, emulsifier, and antimicrobial agent. PHASE WT. % DI Water 78.20% Disodium EDTA 0.80% CARBOPOL 940
Moisturizing Lotion with Laurest 220 and Squalane In this formulation, Laurest 220 acts as an emollient, emulsifier, and antimicrobial agent. PHASE WT. % DI Water 78.20% Disodium EDTA 0.80% CARBOPOL 940
SKIN BRIGHTENING SYSTEM THE POWER OF FOAM AND PHOTODAMAGE O F T H E FA C E. Powerful 3-Piece Regimen Kit
 SKIN BRIGHTENING SYSTEM THE R E V O L U T I O N A RY POWER OF FOAM C L I N I C A L LY P R O V E N T O T R E AT P I G M E N TAT I O N AND PHOTODAMAGE O F T H E FA C E Powerful 3-Piece Regimen Kit Foam drug
SKIN BRIGHTENING SYSTEM THE R E V O L U T I O N A RY POWER OF FOAM C L I N I C A L LY P R O V E N T O T R E AT P I G M E N TAT I O N AND PHOTODAMAGE O F T H E FA C E Powerful 3-Piece Regimen Kit Foam drug
(12) United States Patent
 (12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
(12) United States Patent McElwain USOO62613B1 (10) Patent No.: US 6,261,3 B1 () Date of Patent: Jul. 17, 2001 (54) SKINCREAM (76) Inventor: Elizena A. McElwain, 1 Picnic La., Hardinsburg, KY (US) 4.0143
EpiCeram Topical therapeutic Skin Barrier Emulsion
 EpiCeram Topical therapeutic Skin Barrier Emulsion PEDIAPHARM INC. Date of preparation: August 31, 2010 Summary Product Information: EpiCeram Skin Barrier Emulsion is a steroid-free, fragrance - free,
EpiCeram Topical therapeutic Skin Barrier Emulsion PEDIAPHARM INC. Date of preparation: August 31, 2010 Summary Product Information: EpiCeram Skin Barrier Emulsion is a steroid-free, fragrance - free,
PRODUCT INFORMATION BREVOXYL CREAM
 NAME F THE MEDICINE Brevoxyl contains benzoyl peroxide. PRDUCT INFRMATIN BREVXYL CREAM DESCRIPTIN Benzoyl peroxide is a white powder with the following chemical structure: The compound has anti-bacterial
NAME F THE MEDICINE Brevoxyl contains benzoyl peroxide. PRDUCT INFRMATIN BREVXYL CREAM DESCRIPTIN Benzoyl peroxide is a white powder with the following chemical structure: The compound has anti-bacterial
United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985
 United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985 54 INK RIBBON CASSETTE PROVIDED WITH 56) References Cited AN EMPREGNATION DEVICE U.S. PATENT DOCUMENTS s 2,76,539
United States Patent (19) 11 Patent Number: 4,526,488 Krull 45) Date of Patent: Jul. 2, 1985 54 INK RIBBON CASSETTE PROVIDED WITH 56) References Cited AN EMPREGNATION DEVICE U.S. PATENT DOCUMENTS s 2,76,539
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
SPECIAL TOPIC. Virginia Clinical Research, Inc., Norfolk, VA b. Oregon Dermatology and Research Center, Portland, OR c
 September 2014 611 Volume 13 Issue 9 Copyright 2014 ORIGINAL ARTICLES Journal of Drugs in Dermatology SPECIAL TOPIC An Aqueous Gel Fixed Combination of Clindamycin Phosphate 1.2% and Benzoyl Peroxide 3.75%
September 2014 611 Volume 13 Issue 9 Copyright 2014 ORIGINAL ARTICLES Journal of Drugs in Dermatology SPECIAL TOPIC An Aqueous Gel Fixed Combination of Clindamycin Phosphate 1.2% and Benzoyl Peroxide 3.75%
(12) United States Patent
 US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
The concept of acne cosmetica was introduced
 CURRENT ISSUES & OPINION A re-evaluation of the comedogenicity concept Zoe Diana Draelos, MD, a,b and Joseph C. DiNardo, MS c Winston-Salem and High Point, North Carolina, and Richmond, Virginia Background:
CURRENT ISSUES & OPINION A re-evaluation of the comedogenicity concept Zoe Diana Draelos, MD, a,b and Joseph C. DiNardo, MS c Winston-Salem and High Point, North Carolina, and Richmond, Virginia Background:
DOWSIL 9040 Silicone Elastomer Blend
 Technical Data Sheet FEATURES & BENEFITS Compatible with a variety of lipophilic active ingredients such as fragrances, sunscreens, vitamins, and vitamin derivatives Clear to slightly translucent crosslinked
Technical Data Sheet FEATURES & BENEFITS Compatible with a variety of lipophilic active ingredients such as fragrances, sunscreens, vitamins, and vitamin derivatives Clear to slightly translucent crosslinked
Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use
 9 October 2017 EMA/CHMP/606830/2014 Committee for Human Medicinal Products (CHMP) Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use Draft agreed by
9 October 2017 EMA/CHMP/606830/2014 Committee for Human Medicinal Products (CHMP) Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use Draft agreed by
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
Main types of acne I. Inflammatory types: II. Non inflammatory types:
 What is acne? Acne is perhaps the most common teenagers skin disease, although sometimes it appears in adults too and is characterized by an inflammation of the sebaceous glands. Common acne which affects
What is acne? Acne is perhaps the most common teenagers skin disease, although sometimes it appears in adults too and is characterized by an inflammation of the sebaceous glands. Common acne which affects
Partners in advancing the commitment to healthy, beautiful skin. Skin Care Catalog
 Partners in advancing the commitment to healthy, beautiful skin Skin Care Catalog WHY TOPIX? Partners in advancing the commitment to healthy, beautiful skin For over 30 years, Topix Pharmaceuticals Inc.
Partners in advancing the commitment to healthy, beautiful skin Skin Care Catalog WHY TOPIX? Partners in advancing the commitment to healthy, beautiful skin For over 30 years, Topix Pharmaceuticals Inc.
Int. Cl."... F21V1/06 U.S. C /352; 362/358. References Cited U.S. PATENT DOCUMENTS 3,787,676 l/1974 Korach /352
 United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
CLINDAMYCIN PHOSPHATE-
 CLINDAMYCIN PHOSPHATE- clindamycin phosphate lotion CLINDAMYCIN PHOSPHATE- clindamycin phosphate solution CLINDAMYCIN PHOSPHATE- clindamycin phosphate gel E. & Co. a division of Pharmaceuticals Inc. ----------
CLINDAMYCIN PHOSPHATE- clindamycin phosphate lotion CLINDAMYCIN PHOSPHATE- clindamycin phosphate solution CLINDAMYCIN PHOSPHATE- clindamycin phosphate gel E. & Co. a division of Pharmaceuticals Inc. ----------
United States Patent (19) 11 4,344,932 Gordon 45 Aug. 17, 1982
 United States Patent (19) 11 Gordon 45 Aug. 17, 1982 54 NAIL CLEANSER 2,764,168 9/1956 Herz... 132/73 3,1,048 9/1964 Hollab... 424/6 (75) Inventor: Harry W. Gordon, New York, N.Y. 3,384,592 5/1968 Weems...
United States Patent (19) 11 Gordon 45 Aug. 17, 1982 54 NAIL CLEANSER 2,764,168 9/1956 Herz... 132/73 3,1,048 9/1964 Hollab... 424/6 (75) Inventor: Harry W. Gordon, New York, N.Y. 3,384,592 5/1968 Weems...
(12) United States Patent (10) Patent No.: US 7,585,200 B1
 US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
Represented by: Integrity Ingredients Corporation
 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 AMPHOTERIC
Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 AMPHOTERIC
Sugar based, very efficient emulsifier for PEG-free O/W lotions and creams
 Technical Information TEGO Care CG 90 Sugar based, very efficient emulsifier for PEG-free O/W lotions and creams Intended use O/W emulsifier Benefits at a glance Emulsifier for "natural" O/W emulsions
Technical Information TEGO Care CG 90 Sugar based, very efficient emulsifier for PEG-free O/W lotions and creams Intended use O/W emulsifier Benefits at a glance Emulsifier for "natural" O/W emulsions
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Duac Once Daily 10 mg/g + 50 mg/g Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of gel contains: 10 mg clindamycin as clindamycin
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Duac Once Daily 10 mg/g + 50 mg/g Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of gel contains: 10 mg clindamycin as clindamycin
Demystifying Skin Care for Massage Therapists Chapter 5
 1 Demystifying Skin Care for Massage Therapists Chapter 5 Created by Nina Howard, Founder and Master Trainer Adapted and Edited by Kathryn Myers, CEO Bellanina Insitute BELLANINA INSTITUTE for Skin and
1 Demystifying Skin Care for Massage Therapists Chapter 5 Created by Nina Howard, Founder and Master Trainer Adapted and Edited by Kathryn Myers, CEO Bellanina Insitute BELLANINA INSTITUTE for Skin and
(12) United States Patent
 (12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
(12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
Essential Elements of Rheology Control
 Essential Elements of Rheology Control Richard Giles AkzoNobel Technical Service and Development Manager Europe, Middle East, Africa and India In-cosmetics Barcelona 2015 Outline Today s trends BALANCE
Essential Elements of Rheology Control Richard Giles AkzoNobel Technical Service and Development Manager Europe, Middle East, Africa and India In-cosmetics Barcelona 2015 Outline Today s trends BALANCE
Design, development and evaluation of solid dispersion incorporated transdermal gel of benzoyl peroxide
 2016; 5(7): 13-18 ISSN: 2277-7695 TPI 2016; 5(7): 13-18 2016 TPI www.thepharmajournal.com Received: 01-05-2016 Accepted: 02-06-2016 Jitender Mor Rajinder Mann Bharti Chopra Design, development and evaluation
2016; 5(7): 13-18 ISSN: 2277-7695 TPI 2016; 5(7): 13-18 2016 TPI www.thepharmajournal.com Received: 01-05-2016 Accepted: 02-06-2016 Jitender Mor Rajinder Mann Bharti Chopra Design, development and evaluation
DERIVATIVES - ETHOXYLATED EMULSIFIERS (Nonionic Surfactants)
 DERIVATIVES - ETHOXYLATED EMULSIFIERS (Nonionic Surfactants) Industry End Product or Formulation Lauryl Alcohol Ethoxylates (C12-C14 7EO) Home and Industrial Detergents Fabric Wash Dish Wash Textile Wash
DERIVATIVES - ETHOXYLATED EMULSIFIERS (Nonionic Surfactants) Industry End Product or Formulation Lauryl Alcohol Ethoxylates (C12-C14 7EO) Home and Industrial Detergents Fabric Wash Dish Wash Textile Wash
Jojoba Buttercreme. Description Off white solid with a creamy texture at 25 C Cream base characteristics Melting point: 55 to 70 C ph 8 to 10
 Jojoba Buttercreme Jojoba Buttercreme INCI: Butyrospermum Parkii (Shea) Butter (and) Jojoba Alcohol (and) Potassium Jojobate (and) Simmondsia Chinensis (Jojoba) Butter (and) Propanediol Description Off
Jojoba Buttercreme Jojoba Buttercreme INCI: Butyrospermum Parkii (Shea) Butter (and) Jojoba Alcohol (and) Potassium Jojobate (and) Simmondsia Chinensis (Jojoba) Butter (and) Propanediol Description Off
United States Patent 19
 United States Patent 19 Van Scott et al. (54) DITHRANOL COMPOSITIONS STABILIZED WITH ALPHA HYDROXYACDS Eugene J. Van Scott, 1138 Sewell La., Rydal, Pa. 19046; Ruey J. Yu, 4 76 Inventors: Lindenwold Ave.,
United States Patent 19 Van Scott et al. (54) DITHRANOL COMPOSITIONS STABILIZED WITH ALPHA HYDROXYACDS Eugene J. Van Scott, 1138 Sewell La., Rydal, Pa. 19046; Ruey J. Yu, 4 76 Inventors: Lindenwold Ave.,
United States Patent (19)
 United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
United States Patent (19) Greenspan et al. (4 CLEANING COMPOSITIONS WITH ORANGE OIL 7 Inventors: Douglas H. Greenspan, Louisville; Phillip A. Low, Littleton, both of Colo. - 73) Assignees: D. Greenspan;
The secondary objective was to evaluate the cosmetic properties and its efficacy after 28 days.
 P0090 CLINICAL EVALUATION OF CUTANEOUS SAFETY AND EFFICACY OF A FACE EMULSION CONTAINING MYRTACIN EXTRACT, PP VITAMIN ANS SABAL EXTRACT ON ACNE PRONE SKIN SUBJECTS TREATED BY TOPICAL ANTI ACNE DRUG THERAPY.
P0090 CLINICAL EVALUATION OF CUTANEOUS SAFETY AND EFFICACY OF A FACE EMULSION CONTAINING MYRTACIN EXTRACT, PP VITAMIN ANS SABAL EXTRACT ON ACNE PRONE SKIN SUBJECTS TREATED BY TOPICAL ANTI ACNE DRUG THERAPY.
SEBOREGULATING. Pureskin.
 SEBOREGULATING Pureskin www.provitalgroup.com Pureskin INTRODUCTION Sulphur has been used as a therapeutic agent to treat dermatological conditions from times immemorial. Its keratolytic action is due
SEBOREGULATING Pureskin www.provitalgroup.com Pureskin INTRODUCTION Sulphur has been used as a therapeutic agent to treat dermatological conditions from times immemorial. Its keratolytic action is due
Topical Steroid Therapy. Shireen Velangi Consultant Dermatology Queen Elizabeth Hospital Birmingham UK
 Topical Steroid Therapy Shireen Velangi Consultant Dermatology Queen Elizabeth Hospital Birmingham UK Aim of the Workshop Non Dermatologists (Dermatologists should go to the Professors panel now!) To increase
Topical Steroid Therapy Shireen Velangi Consultant Dermatology Queen Elizabeth Hospital Birmingham UK Aim of the Workshop Non Dermatologists (Dermatologists should go to the Professors panel now!) To increase
EPIDUO GEL PRODUCT INFORMATION
 EPIDUO GEL PRODUCT INFORMATION NAME OF THE MEDICINE EPIDUO Topical gel: 0.1% adapalene + 2.5% benzoyl peroxide Common Names: Adapalene and benzoyl peroxide Chemical Name : - Adapalene: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic
EPIDUO GEL PRODUCT INFORMATION NAME OF THE MEDICINE EPIDUO Topical gel: 0.1% adapalene + 2.5% benzoyl peroxide Common Names: Adapalene and benzoyl peroxide Chemical Name : - Adapalene: 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic
Order of Ingredient Declaration Descending order of predominance
 Order of Ingredient Declaration Descending order of predominance Exceptions... Active drug ingredients Ingredients with less than 1% concentration Color additives "And other ingredients" 21 CFR 701.3(a),
Order of Ingredient Declaration Descending order of predominance Exceptions... Active drug ingredients Ingredients with less than 1% concentration Color additives "And other ingredients" 21 CFR 701.3(a),
Men and women who have or have had blemish-prone skin, and anyone who experiences breakouts.
 Nu Skin Clear Action Clearing beyond the breakout SYSTEM OVERVIEW The Nu Skin Clear Action system focuses on more than just current breakouts. The Nu Skin Clear Action products are designed to work together
Nu Skin Clear Action Clearing beyond the breakout SYSTEM OVERVIEW The Nu Skin Clear Action system focuses on more than just current breakouts. The Nu Skin Clear Action products are designed to work together
Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes
 Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes Chapter 18 (Viscosity-inducing Agents) 1. What is Carbomer NF? 2. Carbomer is commonly referred to
Chapters 18, 22 & 30 Viscosity-inducing Agents, Ointment Bases and Ointments, Creams, Gels, and Pastes Chapter 18 (Viscosity-inducing Agents) 1. What is Carbomer NF? 2. Carbomer is commonly referred to
United States Patent (19)
 United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
Which dissolution media of clindamycin phosphate topical gel
 Which dissolution media of clindamycin phosphate topical gel The Borg System is 100 % Retrievable Which dissolution media of clindamycin phosphate topical gel Learn about Clindagel Topical Gel ( Clindamycin
Which dissolution media of clindamycin phosphate topical gel The Borg System is 100 % Retrievable Which dissolution media of clindamycin phosphate topical gel Learn about Clindagel Topical Gel ( Clindamycin
Rosacea patients form a subset of individuals with
 Optimizing Redness Reduction, Part 1: Rosacea and Skin Care Zoe Diana Draelos, MD Cosmetic Consultation Rosacea patients form a subset of individuals with sensitive skin, which makes the process of selecting
Optimizing Redness Reduction, Part 1: Rosacea and Skin Care Zoe Diana Draelos, MD Cosmetic Consultation Rosacea patients form a subset of individuals with sensitive skin, which makes the process of selecting
Skin knows the difference
 Skin knows the difference Cleanse Protect Treat Moisturize SECURA is a proven four-step preventive skin care system. SECURA products are formulated with high-quality ingredients that define the standard
Skin knows the difference Cleanse Protect Treat Moisturize SECURA is a proven four-step preventive skin care system. SECURA products are formulated with high-quality ingredients that define the standard
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
Hydrolyzed Jojoba Esters to Potentiate Glycerin Moisturization
 Hydrolyzed Jojoba Esters to Potentiate Glycerin Moisturization Tiffany N. Oliphant and Douglas W. Gilmore Floratech, Chandler, AZ, USA Robert A. Harper, PhD Harper and Associates, La Jolla, CA USA KEY
Hydrolyzed Jojoba Esters to Potentiate Glycerin Moisturization Tiffany N. Oliphant and Douglas W. Gilmore Floratech, Chandler, AZ, USA Robert A. Harper, PhD Harper and Associates, La Jolla, CA USA KEY
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O130248A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0130248A1 MOZZOne et al. (43) Pub. Date: (54) TOPICAL ANTI-INFLAMMATORY COMPOSITIONS (76) Inventors: Keith
(19) United States US 2003O130248A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0130248A1 MOZZOne et al. (43) Pub. Date: (54) TOPICAL ANTI-INFLAMMATORY COMPOSITIONS (76) Inventors: Keith
SEBUSPOT. distributor. product profiles β-active product profiles
 β-active SEBUSPOT DESCRIPTION: Environ s β-active Sebuspot is a mild, effective gel with Australian Tea Tree Oil know for its antibacterial properties. It is designed for use on individual blemishes and
β-active SEBUSPOT DESCRIPTION: Environ s β-active Sebuspot is a mild, effective gel with Australian Tea Tree Oil know for its antibacterial properties. It is designed for use on individual blemishes and
United States Patent (19) Garlen
 United States Patent (19) Garlen 11 May 3, 1983 4 METHOD AND COMPOSITION FOR DYENG HUMAN HAIR 7) Inventor: David Garlen, Roselle Park, N.J. (73) Assignee: Michael-David Laboratories, Roselle Park, N.J.
United States Patent (19) Garlen 11 May 3, 1983 4 METHOD AND COMPOSITION FOR DYENG HUMAN HAIR 7) Inventor: David Garlen, Roselle Park, N.J. (73) Assignee: Michael-David Laboratories, Roselle Park, N.J.
TEGO Carbomer 140 G TEGO Carbomer G
 TEGO 140 G TEGO 141 1 G Convenient granulated viscosity adjusters and builders, emulsion stabilizers Granulated s with numerous advantages: lower dusting, easier to process, higher bulk density First s
TEGO 140 G TEGO 141 1 G Convenient granulated viscosity adjusters and builders, emulsion stabilizers Granulated s with numerous advantages: lower dusting, easier to process, higher bulk density First s
GSK Clinical Study Register
 In February 2013, GlaxoSmithKline (GSK) announced a commitment to further clinical transparency through the public disclosure of GSK Clinical Study Reports (CSRs) on the GSK Clinical Study Register. The
In February 2013, GlaxoSmithKline (GSK) announced a commitment to further clinical transparency through the public disclosure of GSK Clinical Study Reports (CSRs) on the GSK Clinical Study Register. The
(12) United States Patent (10) Patent No.: US 7,188,625 B2
 US007188625B2 (12) United States Patent (10) Patent No.: US 7,188,625 B2 Durette (45) Date of Patent: Mar. 13, 2007 (54) OCULAR SURGICAL PROTECTIVE SHIELD 4,024.405 A * 5/1977 Szot... 250,516.1 5,390,373
US007188625B2 (12) United States Patent (10) Patent No.: US 7,188,625 B2 Durette (45) Date of Patent: Mar. 13, 2007 (54) OCULAR SURGICAL PROTECTIVE SHIELD 4,024.405 A * 5/1977 Szot... 250,516.1 5,390,373
Results Clinical Photography
 A High-Strength Retinol Serum Enhanced with N-Acetyl Glucosamine Provides Significant Anti-Aging Effects in Combination with a Comprehensive Skincare Regimen Joel Schlessinger, MD ; Brenda L. Edison, BA
A High-Strength Retinol Serum Enhanced with N-Acetyl Glucosamine Provides Significant Anti-Aging Effects in Combination with a Comprehensive Skincare Regimen Joel Schlessinger, MD ; Brenda L. Edison, BA
United States Patent (19) Frankel
 United States Patent (19) Frankel 11 Patent Number: 45 Date of Patent: Jan. 27, 1987 (54) SWEAT COLLECTING HEADBAND 76) Inventor: Alfred R. Frankel, 403 Gulf Way - Apt. 701, St. Petersburg, Fla. 33706
United States Patent (19) Frankel 11 Patent Number: 45 Date of Patent: Jan. 27, 1987 (54) SWEAT COLLECTING HEADBAND 76) Inventor: Alfred R. Frankel, 403 Gulf Way - Apt. 701, St. Petersburg, Fla. 33706
L A N O L I N LANOLIN NATURE S ORIGINAL SKIN PROTECTION
 L A N O L I N A ND I TS D E RIVATI V ES T he N atu r al Cho i ce www.lanolin.de LANOLIN NATURE S ORIGINAL SKIN PROTECTION OUTLINE NK Ingredients Singapore Lanolin and what it does Product range and how
L A N O L I N A ND I TS D E RIVATI V ES T he N atu r al Cho i ce www.lanolin.de LANOLIN NATURE S ORIGINAL SKIN PROTECTION OUTLINE NK Ingredients Singapore Lanolin and what it does Product range and how
PO Box 5411 Arlington, TX SF A-348
 SF A-348 PRODUCT DESCRIPTION SF A-348 organosilicone is a proprietary copolymer that represents a class of aminosilicone polyalkyleneoxide copolymers for hair conditioning. Conventional organomodified
SF A-348 PRODUCT DESCRIPTION SF A-348 organosilicone is a proprietary copolymer that represents a class of aminosilicone polyalkyleneoxide copolymers for hair conditioning. Conventional organomodified
PCA derivatives. Our performant range of humectants
 PCA derivatives Our performant range of humectants enews August 2015 What is PCA? Pyrrolidone carboxylic Acid (PCA) is one of the component of the Natural Moisturizing Factor (NMF) NMF is the natural moisturizing
PCA derivatives Our performant range of humectants enews August 2015 What is PCA? Pyrrolidone carboxylic Acid (PCA) is one of the component of the Natural Moisturizing Factor (NMF) NMF is the natural moisturizing
(12) United States Patent (10) Patent No.: US 9,468,596 B2
 USOO946896B2 (12) United States Patent () Patent No.: Eizen et al. () Date of Patent: Oct. 18, 2016 (4) MULTI USE COSMETIC FORMULA (2013.01); A61K 8/73 (2013.01); A61K 8/737 (2013.01); A61O 1/00 (2013.01);
USOO946896B2 (12) United States Patent () Patent No.: Eizen et al. () Date of Patent: Oct. 18, 2016 (4) MULTI USE COSMETIC FORMULA (2013.01); A61K 8/73 (2013.01); A61K 8/737 (2013.01); A61O 1/00 (2013.01);
Tolerance of a Low-Level Blue and Red Light Therapy Acne Mask in Acne Patients with Sensitive Skin
 Poster 7098 Tolerance of a Low-Level Blue and Red Light Therapy Acne Mask in Acne Patients with Sensitive Skin Dara Miller 1, Michael J. Cohen 1, Adegboyega Adenaike 1, Julie Biron 2, Michael H. Gold,
Poster 7098 Tolerance of a Low-Level Blue and Red Light Therapy Acne Mask in Acne Patients with Sensitive Skin Dara Miller 1, Michael J. Cohen 1, Adegboyega Adenaike 1, Julie Biron 2, Michael H. Gold,
