(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
|
|
|
- Merry Long
- 5 years ago
- Views:
Transcription
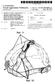
1 (19) United States US A1 (12) Patent Application Publication (10) Pub. No.: US 2009/ A1 Mass0ni et al. (43) Pub. Date: May 7, 2009 (54) AIR OXIDATION HAIR DYE APPLICATION SYSTEMAND METHOD FOR COLORING HAIR USING THE SAME (75) Inventors: Jack T. Massoni, New Fairfield, CT (US); Alan Olsson, Greenwich, CT (US); Peter Mackinson, Wyckoff, NJ (US); Kevin Chan, Lebanon, NJ (US) Correspondence Address: FITZPATRICK CELLAHARPER & SCINTO 30 ROCKEFELLER PLAZA NEW YORK, NY (US) (73) Assignee: COMBE INCORPORATED, White Plains, NY (US) (21) Appl. No.: 11/934,209 (22) Filed: Nov. 2, 2007 Publication Classification (51) Int. Cl. A45D 24/22 ( ) A45D 704 ( ) A61O 5/10 ( ) (52) U.S. Cl /208; 132/112; 132/116 (57) ABSTRACT An air oxidation hair dye application system and a method for coloring hair using this system are provided. The system includes a container holding the hair dye and a comb-shaped applicator mounted on the container
2 Patent Application Publication May 7, 2009 Sheet 1 of 6 US 2009/ A1 E O) (UI 5
3
4 Patent Application Publication May 7, 2009 Sheet 3 of 6 US 2009/ A1 s CN my C 'dus SUIII &U 9
5 Patent Application Publication May 7, 2009 Sheet 4 of 6 US 2009/ A1
6 Patent Application Publication May 7, 2009 Sheet 5 of 6 US 2009/ A1 C5 - seesy CD o
7 Patent Application Publication May 7, 2009 Sheet 6 of 6 US 2009/ A1 : N) V
8 US 2009/ A1 May 7, 2009 AIR OXDATION HARDYE APPLICATION SYSTEMAND METHOD FOR COLORING HAIR USING THE SAME BACKGROUND OF THE INVENTION Field of the Invention The present invention relates to an air oxidation hair dye application system and a method for coloring hair using this system Human hair is treated in diverse ways with various cosmetic preparations. The treatments include, for example, cleansing using shampoos, care and regeneration using rinses and treatments, as well as bleaching, coloring and shaping the hair using colorants, tints, waving compositions and styling preparations. Compositions for changing or nuancing the color of head hair, however, play a prominent role Colorants or tints that comprise so-called direct dyes as the coloring component are typically used for tempo rary colorations. These are dye molecules that attach directly to the hair and require no oxidative process to develop the color. One example of Such a dye is henna, which has been used for hundreds of years to color both body and hair. Direct dyes are typically very sensitive to shampooing, which can be used to remove the dye from the hair For lasting, intense colorations with corresponding fastness properties, the so-called oxidation colorants are often employed. Such colorants typically comprise oxidation dye precursors, so-called developer components and coupler components. Under the influence of oxidizing agents or of atmospheric oxygen (air oxidation dyes), the developer com ponents form the actual dyes with one another or couple with one or more coupler components. The oxidation colorants are characterized by excellent, long-lasting coloring results. For natural-looking colorations, a mixture of a relatively large number of oxidation dye precursors is typically used Oxidative hair colorants are formulated in the form of aqueous emulsions or coloring gels, which, if appropriate, are mixed directly prior to application with a separately for mulated oxidizing agent preparation. Typically, they are packaged in hard or soft dispensers when sold to consumers. Currently available containers and dispensers for oxidative hair dyes, such as the container disclosed in U.S. Pat. No. 7,052,752, are designed to prevent the dyes from oxidizing during storage. However, since air oxidation hair dyes rapidly oxidize upon exposure to air, they can produce undesirable coloring results due to premature oxidation. The known cur rently available containers and dispensing systems are insuf ficient for preventing and/or minimizing such premature oxi dation. SUMMARY OF THE INVENTION The present invention solves a premature oxidation problem that exists in the area of coloring hair using air oxidation hair dye compositions by providing a system and a method for immediate application of the dye to the hair. In particular, the present invention provides a system and method for directly and evenly applying the air oxidation dye to the hair with minimal exposure to air prior to contact with hair This system includes a container, an air oxidation hair dye composition in the container and an applicator mounted on the container. The applicator is in fluid commu nication with the inside of the container and is capable of receiving the dye composition from the container. In particu lar, the applicator is in the form of a comb-like structure that projects from a base and has an internal manifold that receives the dye from the container. A number of comb teeth project sidewardly from the applicator, thereby to define an applica tor comb. Openings for dispensing the dye are formed in the applicator comb, preferably between adjacent comb teeth The container may be any suitable container capable of retaining an air oxidation hair dye composition. Preferably, the container is adapted to minimize premature oxidation of the dye during storage. For ease of application, the container is preferably a tube, which can be squeezed by the consumer to force the dye through the openings in the applicator. Accordingly, the dye is forced from the container through the openings in the applicator Substantially immediately before being applied by the applicator to the hair. Therefore, prema ture oxidation of the dye prior to application to the hair is greatly reduced of minimized The method of coloring hair according to the present invention comprises applying the air oxidation hair dye com position using the above-described system. Preferably, the air oxidation hair dye composition is combed through the hair immediately after and/or during application to ensure unifor mity of the coverage and to achieve better coloring results, and again to minimize or greatly reduce premature oxidation of the dye. DESCRIPTION OF RELATED ART (0010 FIG. 1 is a side, cross-sectional view of an applicator comb structure in accordance with an embodiment of the present invention FIG. 2 is a front view of the applicator comb struc ture in FIG FIG. 3 is a perspective view of the applicator comb structure in FIGS. 1 and FIG. 4A is a back view of part one of an applicator comb structure in accordance with the present invention FIG. 4B is a side view of the first part of the appli cator comb structure in FIG. 4A. (0015 FIG. 4C is a perspective view of the first part of the applicator comb structure in FIGS. 4A and 4B FIG. 5A is a back view of a second part of an appli cator comb structure in accordance with the present inven tion. (0017 FIG. 5B is a side view of the second part of the applicator comb structure in FIG. 5A. (0018 FIG. 5C is a perspective view of the second part of the applicator comb structure in FIGS.5A and 5B. DETAILED DESCRIPTION OF THE INVENTION The present invention is related to an air oxidation hair dye application system and a method for coloring hair using this system. The present inventive system and method mitigate the existing problems associated with premature oxidation of the air oxidation hair dye that occurs when this dye comes into contact with air The system inaccordance with the present invention includes a container, an air oxidation hair dye in the container and an applicator mounted on the container. The applicator is in fluid communication with the inside of the container and is capable of receiving the dye from the container. In particular, the applicator is in the form of a comb structure defining a comb that projects from a base and has an internal manifold
9 US 2009/ A1 May 7, 2009 that receives the dye from the container. A number of comb teeth project sidewardly from the applicator, defining an applicator comb. Openings for dispensing the dye are formed in the applicator comb, preferably between adjacent comb teeth One embodiment of the applicator in accordance with the present invention is shown in FIG.1. Specifically, the applicator 1 has a base 2 and is mounted on a container 3, which holds the air oxidation hair dye composition. In this embodiment, the applicator 2 is mounted on the container 3 via a thread 4. Other types of mounting, Such as using a Snap or an adhesive and the like, are also possible. Alternatively, the base may be molded to or with the container to form a unitary structure The interior of the applicator shown in FIG. 1 is formed with a passage 3a that communicates with the threaded base and opens into a manifold 5 that communicates with separate openings 6, each of which is formed in the applicator between each of the inner ends of the respective comb teeth 7. The diameter of these openings is preferably from about 1.3 mm to about 1.9 mm, more preferable from about 1.5 mm to about 1.7 mm. The openings can have the same diameter or the diameter of each opening may differ, if desired. For example, as shown in FIG. 2, the five lower-most openings 6a-6e, each between a pair of the six lower-most comb teeth, may have a diameter of about 1.5 mm. The next three lower-most openings may have a diameter of about 1.7 mm and the remaining two openings may have a diameter of about 1.5 mm. The sizes of the respective openings between adjacent comb teeth may be determined to facilitate uniform distribution of the dye composition along the entire extent of the applicator comb As shown in FIG. 3, the applicator comb can be molded in two parts 8 and 9. As shown in FIGS. 4A-4C, the first part 8 incorporates the base 2, a first part 10 of the comb back 10a, the comb teeth 7 and one side 11 of the passage from the base 3a and one side of the manifold 12. As shown in FIGS.5A-5C, the other part 9 is a closure for the first part 8 and includes the second part 13 of the comb back 10a, the other side 14 of the passage from the base 3a and the other side of the manifold 15. The two parts 8 and 9 are secured together by, for example, ultrasonic welding to complete the manifold and enclose it as well as the respective openings between adjacent teeth of the comb When the parts 8 and 9 are assembled, the openings between adjacent comb teeth project through a flat Surface that is parallel to the axis of the comb back and essentially perpendicular to both opposing outer side Surfaces of each of the teeth. Opposing surfaces slope from both edges of the flat Surfaces outwardly and toward the comb back. Accordingly, the combined surface between adjacent comb teeth is trap ezoidal in a cross-section taken perpendicular to the axis of the comb back Comb applicators other than those described above can be mounted on the container in accordance with the present invention. Such applicator combs include those dis closed in U.S. Pat. Nos. 6,915,807; 6,588,433; 6,286,518; 6,112,751; 6,065,891; 5,337,764 and 3,446,216, as well as in U.S. Patent Application Publication No. 2005/ A1, which are all incorporated herein by reference In accordance with the present invention, the type of container on which the applicator comb is mounted is not specifically limited, so long as the air oxidation hair dye composition can be expelled from the openings. Preferably, the container is a tube, which can be squeezed to propel the dye into the manifold and through the openings The container is also preferably designed to mini mize the oxidation of the dye during storage. One example of such a container is disclosed in U.S. Pat. No. 7,052,752, which is incorporated herein by reference The air oxidation hair dye application system in accordance with the present invention preferably includes a removable barrier, such as a seal or a cap, which prevents air that can enter the applicator through the openings from com ing into contact with the dye composition before the applica tion of the dye is desired. When the dye is to be applied, this barrier is at least partially removed to allow the dye to be propelled through the openings in the applicator. After the application is complete, the barrier may be replaced to protect the remaining dye composition, if any, from premature oxi dation In order to apply the hair dye in accordance with the present invention, the user would propel the dye through the manifold and the openings onto the hair. The comb may then be drawn through the hair to apply the dye to it in an even manner. Because the dye is essentially first exposed to air when it exits the openings 6 between the comb teeth, which is Substantially immediately prior to application to the hair, in accordance with the present invention, premature oxidation of the dye is greatly reduced or minimized Various air oxidation hair dyes can be placed in the container and applied in accordance with the present inven tion. Suitable dyes include dye intermediates or precursors. Precursors known as primary intermediates' produce colors when oxidized. Another class of precursors, known as "cou plers' or secondary intermediates, form reactive dye spe cies when oxidized in the presence of a primary intermediate but, in general, do not produce any color when oxidized alone. The coupler is utilized to expand the color range by reaction with the primary intermediate, and may also be used to accel erate color formation. Oxidation dye precursors (primary intermediates and couplers) are described, for example, in Sagarin, "Cosmetic Science and Technology'. Interscience, Special Edition, Volume 2, pages 308 to 310; and The Chem istry of Synthetic Dyes', Volume 5, Academic Press, Inc., New York and London (1971) Non-limiting examples of precursors suitable for use herein and which may function as primary intermediates are 1,4-diamino-benzene (p-phenylenediamine); 1,4-di amino-2-methyl-benzene (p-toluylenediamine); 1,4-di amino-2,6-dimethyl-benzene: 1,4-diamino-3,5-diethyl-ben Zene; 1,4-diamino-2,5-dimethyl-benzene, 1,4-diamino-2,3- dimethylbenzene: 2-chloro-1,4-diaminobenzene: 1,4- diamino-2-(thiophen-2-yl)benzene: 1,4-diamino-2- (thiophen-3-yl)benzene: 1,4-diamino-2-(pyridin-3-yl) benzene: 2,5-diaminobiphenyl: 1,4-diamino-2- methoxymethyl-benzene: 1,4-diamino-2- aminomethylbenzene: 1,4-diamino-2-hydroxymethyl benzene: 1,4-diamino-2-(2-hydroxyethoxy)benzene: 2-(2- (acetylamino)ethoxy)-1,4-diaminobenzene: 4-phenylamino aniline: 4-dimethylamino-aniline, 4-diethylamino-aniline; 4-dipropylamino-aniline: 4-ethyl(2-hydroxyethyl)amino aniline: 4-di(2-hydroxyethyl)amino-aniline, 4-di(2-hy droxyethyl)amino-2-methyl-aniline: 4-(2-methoxyethyl) amino-aniline, 4-(3-hydroxypropyl)amino-aniline, 4-(2, 3-dihydroxypropyl)amino-aniline; 1,4-diamino-2-(2- hydroxyethyl)-benzene: 1,4-diamino-2-(1-methylethyl)- benzene; 1,3-bis(4-aminophenyl)(2-hydroxyethyl)amino
10 US 2009/ A1 May 7, propanol; 1,4-bis(4-aminophenyl)amino-butane; 1.8-bis (2,5-diaminophenoxy)-3,6-dioxaoctane, 4-amino-phenol; 4-amino-3-methyl-phenol; 4-amino-3-(hydroxymethyl)- phenol, 4-amino-3-fluoro-phenol, 4-methylamino-phenol; 4-amino-2-(aminomethyl)-phenol, 4-amino-2-(hydroxym ethyl)-phenol, 4-amino-2-fluorophenol, 4-amino-2-(2-hy droxyethyl)-aminomethylphenol, 4-amino-2-methyl-phe nol, 4-amino-2-(methoxymethyl)-phenol, 4-amino-2-(2- hydroxyethyl)-phenol; 5-amino-salicylic acid; 2,5-diamino pyridine; 2,4,5,6-tetramine-pyrimidine; 4,5-diamino-1-1 (2- hydroxyethyl)-1h-pyrazole; 4.5-diamino-1-(1- methylethyl)-1h-pyrazole; 4,5-diamino-1-(4- methylphenyl)methyl)-1h-pyrazole: 1-(4-chlorophenyl) methyl-4,5-diamino-1h-pyrazole; 4,5-diamino-1-methyl 1H-pyrazole; 2-aminophenol; 2-amino-6-methylphenol; and 2-amino-5-methylphenol Non-limiting examples of couplers suitable for use herein are N-(3-dimethylamino-phenyl)-urea; 2,6-diamino pyridine, 2-amino-4-(2-hydroxyethyl)aminoanisole; 2,4- diamino-1-fluoro-5-methylbenzene: 2,4-diamino-1-meth oxy-5-methylbenzene 2,4-diamino-1-ethoxy-5-methyl benzene: 2,4-diamino-1-(2-hydroxyethoxy)-5- methylbenzene: 2,4-di(2-hydroxyethyl)amino-1,5- dimethoxybenzene: 2,3-diamino-6-methoxy-pyridine; 3-amino-6-methoxy-2-(methylamino)pyridine; 2,6-di amino-3,5-dimethoxypyridine; 3,5-diamino-2,6-dimethoxy pyridine, 1,3-diaminobenzene: 2,4-diamino-1-(2-hydroxy ethoxy)benzene: 1,3-diamino-4-(2,3-hydroxypropoxy) benzene: 2,4-diamino-1,5-di(2-hydroxyethoxy)-benzene: 1-(2-aminoethoxy)-2,4-diaminobenzene: 2-amino-1-(2-hy droxyethoxy)-4-methylaminobenzene: 2,4-diaminophe noxyacetic acid ester, 3-di(2-hydroxyethyl)aminoaniline; 4-amino-2-di(2-hydroxyethyl)amino-1-ethoxy-benzene: 5-methyl-2-(1-methylethyl)phenol; 3-(2-hydroxyethyl) aminoaniline; 3-(2-aminoethyl)aminoaniline; 1,3-di(2,4- diaminophenoxy)propane; di(2,4-diaminophenoxy)meth ane, 1,3-diamino-2,4-dimethoxybenzene: 2,6-bis(2- hydroxyethyl)aminotoluene; 4-hydroxyindole; 3-dimethylaminophenol; 3-diethylaminophenol; 5-amino-2- methylphenol; 5-amino-4-fluoro-2-methyl-phenol; 5-amino 4-methoxy-2-methylphenol; 5-amino-4-ethoxy-2-meth ylphenol; 3-amino-2,4-dichlorophenol; 5-amino-2,4- dichlorophenol; 3-amino-2-methyl-phenol; 3-amino-2- chloro-6-methylphenol; 3-aminophenol; 2-(3- hydroxyphenyl)-aminoacetamide; 5-(2-hydroxyethyl) amino-4-methoxy-2-methylphenol; 5-(2-hydroxyethyl) amino-2-methylphenol; 3-(2-hydroxyethyl)amino phenol; 3-(2-methoxyethyl)amino-phenol; 5-amino-2- ethyl-phenol; 5-amino-2-methoxyphenol; 2-(4-amino-2- hydroxyphenoxy)ethanol; 5-(3-hydroxypropyl)amino-2- methylphenol; 3-(2,3-dihydroxypropyl)amino-2- methylphenol; 3-(2-hydroxyethyl)amino-2-methylphenol; 2-amino-3-hydroxypyridine; 5-amino-4-chloro-2-meth ylphenol; 1-naphthol: 2-methyl-1-naphthol: 1,5-dihydrox ynaphthalene; 1,7-dihydroxy-naphthalene: 2,3-dihydrox ynaphthalene, 2.7-dihydroxy-naphthalene, 2-methyl-1- naphthol-acetate; 1,3-dihydroxybenzene: 1-chloro-2,4- dihydroxy-benzene: 2-chloro-1,3-dihydroxybenzene; 1.2- dichloro-2,4-dihydroxy-4-methylbenzene: 1,5-dichloro-2,4- dihydroxy-benzene: 1,3-dihydroxy-2-ethyl-benzene: 3,4- methylenedioxy-phenol; 3.4-methylenedioxy-aniline; 6-bromo-1-hydroxy-3,4-methylenedioxybenzene: 3,4-di aminobenzoic acid; 3,4-dihydroxy-6-hydroxy-1,4(2H)ben Zoxazine; 6-amino-3,4-dihydro-1,4(2H)-benzoxazine; 3-me thyl-1-phenyl-5-pyrazolone; 5,6-dihydroxyindole: 5,6- dihydroxyindoline; 5-hydroxyindole; and 6-hydroxyindole The salt forms of the above-noted dye molecules that form stable salts may also be used. It should also be understood that the precursors described above are by way of example only and are not intended to be exhaustive of oxida tive dyes suitable for use in accordance with the present invention Various commercially available oxidation dyes may be used. Some of these dyes include RODOL 4BXN (2-amino-4-hydroxyethylaminoanisole sulfate); RODOL 2A3PYR (2-amino-3-hydroxypyridine); RODOL 6CP (5-amino-6-chloro-o-cresol); RODOL RED BN (4-hydrox ypropylamino-3-nitrophenol); RODOL PAOX (2-methyl-5- hydroxyethyl aminophenol); RODOL HDAP (4.5 diamino 1-(2-hydroxyethyl)pyrazole sulfate); RODOL 6AMC (6-amino-m-cresol); RODOL PBASE (p-aminophenol cos metic grade); RODOL EG (m-aminophenol); RODOL 2G (o-aminophenol); RODOL BLFX (2.5 diamine toluene sul fate); RODOLERN (1-Naphthol); RODOL RSTECH (resor cinol); RODOL D (p-phenylenediamine); HC YELLOW 4: HC YELLOW 5; HC BLUE 2 CP; RODOL BROWN 2R (2-nitro-p-phenylenediamine); RODOL 4A3NP (4-amino-3- nitrophenol); RODOL BROWN SO (2-chloro-p-phenylene diamine sulfate); RODOL CRS (4-Chlororesorcinol): RODOL 2MR (2-methylresorcinol high purity); RODOL GRAY BS (n-phenyl-p-phenylenediamine sulfate); RODOL PMP (1-phenyl-3-methyl-5-pyrazolone); RODOL PAOC (4-amino-2-hydroxytoluene); RODOL3M4AP (4-amino-mcresol); RODOL GRAY HED (N.N-bis-hydroxyethyl-p-phe nylenediamine sulfate); RODOL PS (p-aminophenol sul fate); RODOL EGS (m-aminophenol sulfate); RODOL DS (p-phenylenediamine sulfate); RODOL MPDS (m-phe nylenediamine sulfate); RODOL 4.JP (4-nitro-o-phenylene diamine); RODOL 9R BASE (2-amino-6-chloro-4-nitrophe nol); RODOL PM (p-methylaminophenol sulfate); RODOL 2,4-DAPE (2.4 diaminophenoxyethanol dihydrochloride); JAROCOL DPE (HSO) (2,4-diaminophenoxyethanol sul fate); and any combination thereof. In particular, Suitable combinations of a base, a precursor and a colorant are selected. 0035) To prevent premature oxidation, it is typical for the dye composition to further comprise one or more antioxi dants. Examples of antioxidants that can be used in accor dance with the present invention include erythorbic acid and sodium sulfite The carrier component comprises water, and, optionally, one or more cosmetically acceptable solvents or diluents, for example the cosmetically acceptable alcohols and ethers, provided that such solvents and diluents are mis cible with water and do not undesirably react with the other materials present in the developer component Preferably, the air oxidation dye composition in accordance with the present invention comprises one or more surfactants. Surfactants aid in the uniform distribution of the colorant composition on the hair Surface and assist the user in rinsing the colorant composition from the hair Subsequent to treatment. Various types of Surfactants can be used Examples of cationic surfactants include amine based Surfactants, such as alkyl amines, alkylethoxy amines, ethoxylated alkyl amines, alkyl alkanol amines and cinna mido alkyl amine cationic quaternary salts, such as cinnami dopropyl trimethyl ammonium chloride. The term amines' includes primary, secondary, tertiary and quaternary amines.
11 US 2009/ A1 May 7, 2009 Other cationic Surfactant may be amidoamines, including C-C alkyl or alkylethoxy mono, di and higher (poly)ami doamines, which can be ethoxylated or unethoxylated. Non limiting examples of Such cationic Surfactants are sodium dimethylaminopropyl coco-aspartamide, cocoamidopropyl dimethylamine, olivamidopropyl dimethylamine, soyami dopropyl dimethylamine, tallowamidopropyl dimethy lamine, and Stearamidoethyl dimethylamine Another class of surfactant that is suitable for use in the present invention is nonionic Surfactants. This class includes long chain (C-C) fatty alcohols, mono, di and triglycerides and their derivatives, and alcohol ethoxylates. Non-limiting examples include steareth 20, oleth 10, laureth 4. PEG-12 glyceryl dioleate, glycerol stearate, sorbitan ole ate, and PPG-9 buteth Yet another suitable class of surfactants is anionic Surfactants. This class includes alkyl and alkyl ether Sulfates Preferably, the surfactants are very mild, skin friendly and derived from natural ingredients. For example, coco glucoside, which is derived from coconut oil and fruit Sugar, can act as an anionic Surfactant and also Smooth the hair structure to improve hair manageability To provide the alkaline conditions desired to pro mote oxidative dyeing, the compositions of this invention may further comprise at least one additional agent that pro vides an alkalizing effect. Examples of such alkalizing agents include, but are not limited to, ammonium hydroxide, alkali metal hydroxides and alkaline earth metal hydroxides: amines, such, alkanolamines, polyalkylene amines, heterocy clic amines; basic amino acids; and the like. Non-limiting examples of Suitable alkalizing agents include ammonium hydroxide, Sodium hydroxide, potassium hydroxide, magne sium hydroxide, calcium hydroxide, urea, ethylamine, dipro pylamine, triethylamine, 1,3-diaminopropane, monoethano lamine, diethanolamine, triethanolamine, aminomethyl propanol, dimethylaminoethanol, diethylenetriamine, mor pholine, diethylaminoethanol, aminoalkylpropanediol. L-arginine, lysine, oxylysine, and histidine Desirably, the air oxidation hair dye compositions further comprise at least one thickening or gelling agent. Long chain fatty alcohols having up to about 22 carbonatoms in the long fatty chain can be thickener constituents in the compositions of this invention. Non-limiting examples of such fatty alcohols are lauryl alcohol, oleyl alcohol, myristyl alcohol, stearyl alcohol, and the like. Mixtures of fatty alco hols are also useful and are commercially available from numerous Suppliers. 0044) Thickening agents suitable for use herein may also be selected from long chain fatty acids having up to about 22 carbon atoms in the long fatty chain thereof. Non-limiting examples of Such long chain fatty acids include oleic acid, stearic acid, myristic acid and linoleic acid. Mixtures offatty acids are also useful and are commercially available from numerous Suppliers The fatty alcohols and fatty acids described above may be in alkoxylated form. Suchalkoxylates may contain an average of one to three, more particularly one to two, alkylene oxide, preferably ethylene oxide, units Other thickening or gelling agents. Such as car bomers, conventionally used in hair coloring compositions may be present as optional ingredients in the compositions of this invention The air oxidation hair dye compositions in accor dance with the present invention can include one or more chelating agents. The term chelating agent (or chelant or "sequestering agent') is well known in the art and refers to a molecule or a mixture of different molecules each capable of forming a chelate with a metal ion. A chelate is an inorganic complex in which a compound (chelant) is coordinated to a metalion at two or more points so that there is a ring of atoms including the metals. Chelants contain two or more electron donoratoms that form coordination bonds with the metalion As used herein, the term chelant includes all salts and derivatives comprising the same functional structure as the parent chelant they are referring to that have similar or better chelating properties. The term "derivatives also includes chelating Surfactant compounds (chelants modi fied to bear a surfactant moiety while keeping the same chelating functionality, see U.S. Pat. No. 5, ). The term "derivatives also includes large molecules comprising one or more chelating groups having the same functional structure as the parent chelants. An example of these large molecules is polymeric EDDS (ethylenediaminedisuccinic acid) Specific chelants that can be used include carboxy lic acids (in particular aminocarboxylic acids), phosphonic acids (in particular aminophosphonic acids), and polyphos phoric acids (in particular linear polyphosphoric acids), their salts and derivatives Carboxylic acid chelants as defined herein are chelants having at least one carboxylic acid moiety ( COOH). Examples of aminocarboxylic acid chelants suit able for use herein include nitrilotriacetic acid and polyami nocarboxylic acids such as diethylenetriamine pentaacetic acid (DTPA), ethylenediamine disuccinic acid (EDDS), eth ylenediamine diglutaric acid (EDGA), 2-hydroxypropylene diamine disuccinic acid (HPDS), glycinamide-n,n'-disuc cinic acid (GADS), ethylenediamine-n- N'-diglutaric acid (EDDG), 2-hydroxypropylenediamine-N- N'-disuccinic acid (HPDDS), ethylenediaminetetraacetic acid (EDTA), dipicolinic acid (DPA), salts thereof and derivatives thereof. An example of a salt that can be used in the hair dye compo sition in accordance with the present invention is trisodium EDTA Other suitable aminocarboxylic chelants for use herein are iminodiacetic acid derivatives. Such as N-2-hy droxyethyl N,N diacetic acid or glyceryl imino diacetic acid (described in EP and EP ), iminodiacetic acid-n'-hydroxypropylsulfonic acid and aspartic acid N-car boxymethyl N-2-hydroxypropyl-3-sulfonic acid (described in EP ), alanine-n,n'-diacetic acid, aspartic acid N,N'-diacetic acid, aspartic acid-n-monoacetic acid and imi nodisuccinic acid chelants (described in EP ), etha noldiglycine acid, salts thereof and derivatives thereof Preferred aminocarboxylic chelants are diamine-n, N'-dipolyacid and monoamine monoamide-n,n'-dipolyacid chelants, salts thereof and derivatives thereof. Preferred poly acids contain at least two acid groups independently selected from the carboxylic acid group (-COOH), sulfonic group ( SOH), the o-hydroxyphenyl group, the m-hydroxyphe nyl group and the p-hydroxyphenyl group. Suitable polyacids include diacids, triacids and tetraacids, preferably diacids. Preferred salts include alkali metal, alkaline earth, ammo nium or Substituted ammonium salts. Exemplary diamine dipolyacids suitable for use 0053 herein include ethylenediamine-n,n'-disuccinic acid
12 US 2009/ A1 May 7, 2009 (EDDS), ethylenediamine-n,n'-diglutaric acid (EDDG), 2-hydroxypropylenediamine-N,N'-disuccinic acid (HP DDS), all disclosed in EP , ethylenedicysteic acid (EDC), disclosed in U.S. Pat. No. 5,693,854, diaminoalkyldi (sulfosuccinic acids) (DDS) disclosed in U.S. Pat. No. 5,472, 642 and EDDHA (ethylenediamine-n-n'-bis(ortho-hy droxyphenyl acetic acid). A specific monoamine that can be used in the present invention is monoamide-n,n'-dipolyacid is glycinamide-n,n'-disuccinic acid (GADS), described in U.S. Pat. No. 4,983,315. EXAMPLE 0054 An experiment was conducted to compare the col oring achieved by applying an air oxidation hair dye compo sition from the container with a comb application in accor dance with the present invention and by applying the same oxidizing dye in a conventional manner. In this experiment, hair coloring was performed under controlled conditions using three air oxidation hair dye compositions on 100% gray laboratory hair. The air oxidation dye compositions used are shown in Tables 1-3. Ingredient TABLE 1. Light Brown Percent by Weight Water Antioxidant O.15 Antioxidant Preservative O.10 Chelating Agent O.2O Anionic Surfactant 3.00 Carbomer O.90 Fragrance OSO Thickeneri Gelling Agent 2.OO Cationic Surfactant O.O1 Air Oxidation Hair Dye Mixture (1,2, benzenetriol solution (solvents: isopropyl alcohol, isopropyl acetate, water and Sulfuric acid); p-phenylenediamine; N,N-bis(2- hydroxyethyl)-p-phenylenediamine Sulfate; p-aminophenol; 2,4-diaminophenoxy ethanol Sulfate; and 2-methyl-5- hydroxyethylaminophenol) Ingredient TABLE 2 Medium Brown Percent by Weight Water 89.45S Antioxidant O.1SO Preservative Antioxidant O.100 Chelating Agent O.200 Anionic Surfactant 3.OOO Carbomer O.9SO Fragrance OSOO Thickeneri Gelling Agent 2.OOO Cationic Surfactant O.O10 Air Oxidation Hair Dye Mixture (1,2,4-benzenetriol 3.63S Solution (solvents: isopropyl alcohol, isopropyl acetate, water and Sulfuric acid);p-phenylenediamine; N,N-bis(2-hydroxyethyl)-p-phenylenediamine sulfate; p-aminophenol and 2-methyl-5- hydroxyethylaminophenol) Ingredient TABLE 3 Dark Brown Percent by Weight Water Antioxidant O.15 Antioxidant Preservative O.10 Chelating Agent O.2O Anionic Surfactant 3.00 Carbomer 1.OO Fragrance OSO Thickeneri Gelling Agent 3.10 Cationic Surfactant O.O1 Air Oxidation Hair Dye Mixture (1,2,4-841 benzenetriol Solution (solvents: isopropyl alcohol, isopropyl acetate, water and Sulfuric acid); p-phenylenediamine; N,N-bis(2- hydroxyethyl)-p-phenylenediamine Sulfate p-aminophenol; 2-methyl-5- hydroxyethylaminophenol; 2-amino-4- hydroxyethylaminoanisole Sulfate) 0055 Each hair dye composition was applied to the hair from the container in accordance with the present invention through the applicator comb, such as that shown in FIGS. 1-5C with all openings having the same diameter, so that the dye came into contact with the hair with no delay. Separately, each hair dye was applied to the hair after a 5 minute and a 10 minute delay. These delays represent the amount of time typically needed to apply an air oxidation hair dye composi tion from conventional containers without the comb applica tor of the present invention. In all cases, the dye was allowed to develop on the hair for 5 minutes, followed by a rinse, shampoo and blow dry. Furthermore, dying under each con dition was repeated The color of the swatches was recorded using a Minolta 508d Spectrophotometer using the Hunter L, a, b scale. "L' is a measure of lightness and varies from 100 for perfect white to zero for perfect black. a is a measure of redness, when the value of a is positive, and a measure of greenness, when negative. b' is a measure of yellowness, when the value of b' is positive, and a measure of blueness, when negative. The results from this experiment are shown below in Table 4. TABLE 4 Sample 'L' sa b Untreated White Hair Light Brown No delay, Swatch O 3.1 No delay, Swatch minute delay, Swatch minute delay, Swatch minute delay, Swatch S.1 10 minute delay, Swatch Medium Brown No delay, Swatch No delay, Swatch O minute delay, Swatch O 5 minute delay, Swatch minute delay, Swatch minute delay, Swatch 2 52.O
13 US 2009/ A1 May 7, 2009 TABLE 4-continued Sample 'L' sa b Dark Brown No delay, Swatch No delay, Swatch minute delay, Swatch minute delay, Swatch minute delay, Swatch 1 SO.2 O.9 S.1 10 minute delay, Swatch O.9 S Based on the L., a, b scale, the differences in light ness are apparent to the human eye at a change of about 0.5. Therefore, according to the results shown in Table 4, it is readily evident that both the 5 and 10 minute delay between dispensing and applying to the hair the air oxidation hair dye composition produce visually lighter, i.e., inferior, results. Therefore, application of an air oxidation hair dye composi tion in accordance with the present invention produces mark edly Superior hair coloring results compared with conven tional air oxidation hair dye application While the invention has been described in conjunc tion with the detailed description thereof and the accompa nying figures, the foregoing description is intended to illus trate and not limit the scope of the invention, which is defined by the scope of the appended claims. Other aspects, advan tages, and modifications are within the scope of the following claims. What is claimed is: 1. An air oxidation hair dye application system comprising: a container; an air oxidation hair dye composition inside the container; and an applicator mounted on the container, wherein the applicator is in fluid communication with the inside of the container and is capable of receiving the air oxidation hair dye composition from the container, and wherein the applicator is in a form of a comb that projects from a base and has an internal manifold that receives the air oxidation hair dye composition from the con tainer and a plurality of openings through which the air oxidation hair dye composition is dispensed from the applicator. 2. The system according to claim 1, wherein the applicator is detachably mounted on the container. 3. The system according to claim 2, wherein the applicator is detachably mounted on the container via a thread. 4. The system according to claim 1, wherein the comb comprises comb teeth and wherein the openings for dispens ing the air oxidation hair dye composition are formed between adjacent comb teeth. 5. The system according to claim 4, wherein the comb is formed with a flat surface that is parallel to a longitudinal axis of the applicator and substantially perpendicular to both opposing outer side Surfaces of each comb tooth, and wherein the openings project through said flat surface. 6. The system according to claim 5, wherein the opposing outer side surfaces slope outwardly and toward a back of the applicator, so that a combined surface between adjacent comb teeth is trapezoidal in a cross-section taken perpendicular to the longitudinal axis of the applicator. 7. The system according to claim 1, wherein the openings are substantially circular and have a diameter from about 1.3 mm to about 1.9 mm. 8. The system according to claim 1, wherein each of the openings has the same diameter. 9. The system, according to claim 1, wherein the container is a tube. 10. A method for coloring hair comprising: providing a container with an air oxidation hair dye com position inside the container and an applicator mounted on the container, wherein the applicator is in fluid com munication with the inside of the container and is capable of receiving the air oxidation hair dye compo sition from the container, and wherein the applicatoris in a form of a comb that projects from a base and has an internal manifold that receives the air oxidation hair dye composition from the container and a plurality of open ings through which the air oxidation hair dye composi tion is dispensed from the applicator, and applying the air oxidation hair dye composition to the hair through the openings in the applicator. 11. The method according to claim 10, further comprising a step of brushing the air oxidation hair dye composition through the hair using the comb. 12. The method according to claim 11, comprising apply ing the air oxidation hair dye composition to the hair while combing the hair with the applicator. c c c c c
5,409,502 4/1995 Braun dye components. The additional dye components can be
 USOO56371.A United States Patent (19) 11 Patent umber: 5,637,1 Balzer et al. Date of Patent: Jun., 1997 54 OXDATIO HAIR DYE COMPOSITIOS OTHER PUBLICATIOS COTAIIG DEVELOPERS, COUPLERS, AD AZODYES Hair Dyes,
USOO56371.A United States Patent (19) 11 Patent umber: 5,637,1 Balzer et al. Date of Patent: Jun., 1997 54 OXDATIO HAIR DYE COMPOSITIOS OTHER PUBLICATIOS COTAIIG DEVELOPERS, COUPLERS, AD AZODYES Hair Dyes,
MANUAL DATA SAFETY (according regulation 76/768/CE)
 KINCREM PRESTIGE CRK+ COSMETIC BEAUTY COLOURING 1.- PRODUCT & COMPANY IDENTIFICATION Product: KINCREM PRESTIGE CRK+ COSMETIC BEAUTY COLOURING (all tones) Company: KIN COSMÈTICS, S.A.U. Pol. Industrial
KINCREM PRESTIGE CRK+ COSMETIC BEAUTY COLOURING 1.- PRODUCT & COMPANY IDENTIFICATION Product: KINCREM PRESTIGE CRK+ COSMETIC BEAUTY COLOURING (all tones) Company: KIN COSMÈTICS, S.A.U. Pol. Industrial
(Hair Colorant (Permanent, Oxidative Type) Type 1: two or three components Oxidative part)
 TECHNICAL INFORMATION SHEET FINISHED COSMETIC PRODUCT TYPE OF COSMETIC PRODUCT (Hair Colorant (Permanent, Oxidative Type) Type 1: two or three components Oxidative part) PRODUCT AND MANUFACTURER IDENTIFICATION
TECHNICAL INFORMATION SHEET FINISHED COSMETIC PRODUCT TYPE OF COSMETIC PRODUCT (Hair Colorant (Permanent, Oxidative Type) Type 1: two or three components Oxidative part) PRODUCT AND MANUFACTURER IDENTIFICATION
COMMISSION REGULATION (EU)
 EN L 315/34 Official Journal of the European Union 26.11.2013 COMMISSION REGULATION (EU) No 1197/2013 of 25 November 2013 amending Annex III to Regulation (EC) No 1223/2009 of the European Parliament and
EN L 315/34 Official Journal of the European Union 26.11.2013 COMMISSION REGULATION (EU) No 1197/2013 of 25 November 2013 amending Annex III to Regulation (EC) No 1223/2009 of the European Parliament and
(12) United States Patent
 USOO9474704B2 (12) United States Patent Mass0ni et al. (10) Patent No.: (45) Date of Patent: US 9.474,704 B2 *Oct. 25, 2016 (54) GRADUAL HAIRCOLOR COMPOSITIONS AND METHODS OF USING THE SAME (71) Applicant:
USOO9474704B2 (12) United States Patent Mass0ni et al. (10) Patent No.: (45) Date of Patent: US 9.474,704 B2 *Oct. 25, 2016 (54) GRADUAL HAIRCOLOR COMPOSITIONS AND METHODS OF USING THE SAME (71) Applicant:
Material Safety Data Sheet
 1. Product and Company Identification 1.1 Commercial product name SALERM COSMETICS Chemical characterization: (preparation) 1.2 Information manufacturer / supplier Company information SALERM COSMETICA
1. Product and Company Identification 1.1 Commercial product name SALERM COSMETICS Chemical characterization: (preparation) 1.2 Information manufacturer / supplier Company information SALERM COSMETICA
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150343112A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0343112 A1 Dyar et al. (43) Pub. Date: (54) LIQUID BANDAGE Publication Classification (71) Applicant: PROBLEM
(19) United States US 20150343112A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0343112 A1 Dyar et al. (43) Pub. Date: (54) LIQUID BANDAGE Publication Classification (71) Applicant: PROBLEM
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
US 20060104928A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0104928A1 Furtado (43) Pub. Date: May 18, 2006 (54) THERMAL HAIR STRAIGHTENING AND (52) U.S. Cl.... 424f702
(12) United States Patent (10) Patent No.: US 6,752,627 B2
 USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
USOO6752627B2 (12) United States Patent (10) Patent No.: US 6,752,627 B2 Lin (45) Date of Patent: Jun. 22, 2004 (54) LIGHT EMITTING TOOTH BRUSH HAVING 5,306,143 A * 4/1994 Levy... 433/29 WHITENING AND
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0107975A1 Bender US 2004O107975A1 (43) Pub. Date: Jun. 10, 2004 (54) EYE MAKEUPSTENCIL (76) Inventor: Beth Bender, New York,
MATERIAL SAFETY DATA SHEET Finished Product.
 MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Clairol Nice n Easy Colorblend Foam This product is sold as a three component
MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Clairol Nice n Easy Colorblend Foam This product is sold as a three component
MATERIAL SAFETY DATA SHEET Finished Product.
 MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Koleston Espuma Color-Intense This product is sold as a three component
MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Koleston Espuma Color-Intense This product is sold as a three component
(12) United States Patent (10) Patent No.: US 6,308,717 B1
 USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
USOO63O8717B1 (12) United States Patent (10) Patent No.: US 6,308,717 B1 Vrtaric (45) Date of Patent: Oct. 30, 2001 (54) HAIR BRUSH WITH MOVABLE BRISTLES 5,657,775 8/1997 Chou... 132/125 5,715,847 * 2/1998
Material Safety Data Sheet
 1. Product and Company Identification 1.1 Commercial product name Chemical characterization: (preparation) 1.2 Information manufacturer / supplier Company information SALERM COSMETICA PROFESIONAL, S.A.
1. Product and Company Identification 1.1 Commercial product name Chemical characterization: (preparation) 1.2 Information manufacturer / supplier Company information SALERM COSMETICA PROFESIONAL, S.A.
Memorandum on hair dye Chemical Sensitisation
 Scientific Committee on Consumer Safety SCCS Memorandum on hair dye Chemical Sensitisation The SCCS adopted this memorandum at its 18 th Plenary meeting of 26 February 2013 About the Scientific Committees
Scientific Committee on Consumer Safety SCCS Memorandum on hair dye Chemical Sensitisation The SCCS adopted this memorandum at its 18 th Plenary meeting of 26 February 2013 About the Scientific Committees
Official Journal of the European Union
 L 36/12 EN COMMISSION REGULATION (EU) 2017/237 of 10 February 2017 amending Annex III to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products (Text with EEA relevance)
L 36/12 EN COMMISSION REGULATION (EU) 2017/237 of 10 February 2017 amending Annex III to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products (Text with EEA relevance)
Tomura, Kazuyo Osaka-shi, Osaka-fu (JP)
 * OJII Eur Pean Patent Office mi mi ii mi 111 ii mi 111 iii Office eurodeen des brevets (11) E P 0 7 1 6 8 4 6 A 1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51 ) nt. CI.6: A61 K 7/1 3
* OJII Eur Pean Patent Office mi mi ii mi 111 ii mi 111 iii Office eurodeen des brevets (11) E P 0 7 1 6 8 4 6 A 1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51 ) nt. CI.6: A61 K 7/1 3
(12) United States Patent (10) Patent No.: US 6,695,888 B2
 USOO6695888B2 (12) United States Patent (10) Patent No.: Bartolone et al. () Date of Patent: Feb. 24, 2004 (54) TRANSITION METAL COMPLEXES AS DYE FOREIGN PATENT DOCUMENTS SNSSYSTs IN HAIR COLORING DE 19852
USOO6695888B2 (12) United States Patent (10) Patent No.: Bartolone et al. () Date of Patent: Feb. 24, 2004 (54) TRANSITION METAL COMPLEXES AS DYE FOREIGN PATENT DOCUMENTS SNSSYSTs IN HAIR COLORING DE 19852
Supplemental January 2009
 Supplemental January 2009 Editor s note: The Surfactant Spectator is always looking for articles that are of interest to our readers. No topic is more interesting to our readers than green surfactants,
Supplemental January 2009 Editor s note: The Surfactant Spectator is always looking for articles that are of interest to our readers. No topic is more interesting to our readers than green surfactants,
COMMISSION DIRECTIVE 2009/36/EC
 17.4.2009 Official Journal of the European Union L 98/31 DIRECTIVES COMMISSION DIRECTIVE 2009/36/EC of 16 April 2009 amending Council Directive 76/768/EEC, concerning cosmetic, for the purpose of adapting
17.4.2009 Official Journal of the European Union L 98/31 DIRECTIVES COMMISSION DIRECTIVE 2009/36/EC of 16 April 2009 amending Council Directive 76/768/EEC, concerning cosmetic, for the purpose of adapting
Safety Data Sheet. according to ANSI Z
 Guidechem Chemical Network - Chemical Manufacturers,suppliers, Safety Data Sheet MSDS 2 of 3 Page 1 of 6 1. Product and Company Identification 1.1 Commercial product name / NOTE: This list is not inclusive
Guidechem Chemical Network - Chemical Manufacturers,suppliers, Safety Data Sheet MSDS 2 of 3 Page 1 of 6 1. Product and Company Identification 1.1 Commercial product name / NOTE: This list is not inclusive
United States Patent (19)
 United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
United States Patent (19) USOO5515542A 11 Patent Number: 5,515,542 Simmons 45) Date of Patent: May 14, 1996 (54) TATTOO-LIKE EFFECT APPAREL 4,546,493 10/1985 Bortnick. 4,642,250 2f1987 Spector... 2,67
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
(19) United States US 2015O157057A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0157057 A1 TRUONG (43) Pub. Date: Jun. 11, 2015 (54) ADJUSTABLE COLLAR STAY FOR MEN AND (52) U.S. Cl. WOMENS
MATERIAL SAFETY DATA SHEET Finished Product.
 MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Balsam Lasting Color - Liquid This product is sold as a three component
MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Balsam Lasting Color - Liquid This product is sold as a three component
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(19) United States US 2005O198829A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0198829 A1 Gray et al. (43) Pub. Date: Sep. 15, 2005 (54) SHAVING RAZOR WITH TRIMMING BLADE (76) Inventors:
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
US 20090131977A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0131977 A1 ROSS (43) Pub. Date: May 21, 2009 (54) COMBINATION TWEEZER AND EYE HAIR Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
(19) United States US 20150117931A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0117931 A1 Jung et al. (43) Pub. Date: (54) FOAM HAVING IMPROVED FEELING (30) Foreign Application Priority
Ref. 11. (12) Patent Application Publication (10) Pub. No.: US 2012/ A1. (19) United States. Polstein et al. (43) Pub. Date: Jun.
 (19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(19) United States US 2012O159696A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0159696A1 Polstein et al. (43) Pub. Date: Jun. 28, 2012 (54) METHOD AND DEVICE FOR PROVIDING AN OPENING ON
(12) United States Patent
 USOO9639 120B2 (12) United States Patent W (10) Patent No.: (45) Date of Patent: May 2, 2017 (54) HEAT DISSIPATIONSTRUCTURE OF WEARABLE ELECTRONIC DEVICE (71) Applicant: ASIA VITAL COMPONENTS CO., LTD.,
USOO9639 120B2 (12) United States Patent W (10) Patent No.: (45) Date of Patent: May 2, 2017 (54) HEAT DISSIPATIONSTRUCTURE OF WEARABLE ELECTRONIC DEVICE (71) Applicant: ASIA VITAL COMPONENTS CO., LTD.,
United States Patent (19) Garlen
 United States Patent (19) Garlen 11 May 3, 1983 4 METHOD AND COMPOSITION FOR DYENG HUMAN HAIR 7) Inventor: David Garlen, Roselle Park, N.J. (73) Assignee: Michael-David Laboratories, Roselle Park, N.J.
United States Patent (19) Garlen 11 May 3, 1983 4 METHOD AND COMPOSITION FOR DYENG HUMAN HAIR 7) Inventor: David Garlen, Roselle Park, N.J. (73) Assignee: Michael-David Laboratories, Roselle Park, N.J.
MATERIAL SAFETY DATA SHEET Finished Product.
 MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Clairol Professional Premium Crème DEMI Baseline Shades: 1N Neutral Black
MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Clairol Professional Premium Crème DEMI Baseline Shades: 1N Neutral Black
Material Safety Data Sheet
 1. Product and Company Identification 1.1 Commercial product name SALERM COSMETICS Chemical characterization: (preparation) 1.2 Information manufacturer / supplier Company information SALERM COSMETICA
1. Product and Company Identification 1.1 Commercial product name SALERM COSMETICS Chemical characterization: (preparation) 1.2 Information manufacturer / supplier Company information SALERM COSMETICA
US HAIR DYEING OR COLORING PATENTS GRANTED IN Title
 US HAIR DYEING OR COLORING PATENTS GRANTED IN 2006 US Patent No. 7,153,494 Dibenzoylmethane sunscreen compositions photostabilized with amphiphilic block copolymers 7,153,331 Dyeing composition for keratinous
US HAIR DYEING OR COLORING PATENTS GRANTED IN 2006 US Patent No. 7,153,494 Dibenzoylmethane sunscreen compositions photostabilized with amphiphilic block copolymers 7,153,331 Dyeing composition for keratinous
WWWWW. ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / A1. 19 United States
 THE MAIN TEA ETA AITOR A TT MA N ALUMINIUM TIN US 20170266826A1 19 United States ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / 0266826 A1 Kole et al. ( 43 ) Pub. Date : Sep. 21, 2017
THE MAIN TEA ETA AITOR A TT MA N ALUMINIUM TIN US 20170266826A1 19 United States ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / 0266826 A1 Kole et al. ( 43 ) Pub. Date : Sep. 21, 2017
(12) United States Patent
 US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
US009491978B2 (12) United States Patent Kim (10) Patent No.: (45) Date of Patent: *Nov. 15, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) (58) HAIR EXTENSION Applicant: Chade Fashions, Inc.,
Int. Cl."... F21V1/06 U.S. C /352; 362/358. References Cited U.S. PATENT DOCUMENTS 3,787,676 l/1974 Korach /352
 United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
United States Patent (19) Tang 54 (75) 73 (21) (22) 51 (52) (58) (56) COLLAPSIBLE LAMPSHADE ASSEMBLY, AND METHOD OF USE Inventor: Yong Tang, Montebello, Calif. Assignee: Sun Housewares, Inc., Los Angeles,
(12) United States Patent (10) Patent No.: US 7434,929 B2
 US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
US007434929B2 (12) United States Patent (10) Patent No.: US 7434,929 B2 JacksOn (45) Date of Patent: Oct. 14, 2008 (54) SWEAT LINER FOR GLASSES D354,970 S 1, 1995 Bole D365,593 S 12/1995 Leonardi (76)
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 US 2015O164752A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0164752 A1 Vena et al. (43) Pub. Date: (54) HAIR DYE COMPOSITION AND METHOD Publication Classification FOR
US 2015O164752A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0164752 A1 Vena et al. (43) Pub. Date: (54) HAIR DYE COMPOSITION AND METHOD Publication Classification FOR
(12) Patent Application Publication (10) Pub. No.: US 2002/ A1
 US 20020029.429A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0029429 A1 DIAS et al. (43) Pub. Date: (54) HAIR COLORING COMPOSITIONS (30) Foreign Application Priority
US 20020029.429A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0029429 A1 DIAS et al. (43) Pub. Date: (54) HAIR COLORING COMPOSITIONS (30) Foreign Application Priority
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 2011 0052520A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0052520 A1 Nguyen et al. (43) Pub. Date: (54) USE OF A COMPOSITION AND PROCESS INVOLVING THE USE OFA NON-HYDROXDE
(19) United States US 2011 0052520A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0052520 A1 Nguyen et al. (43) Pub. Date: (54) USE OF A COMPOSITION AND PROCESS INVOLVING THE USE OFA NON-HYDROXDE
ANNEX VI LIST OF PRESERVATIVES WHICH COSMETIC PRODUCTS MAY CONTAIN
 ANNEX VI LIST OF PRESERVATIVES WHICH COSMETIC PRODUCTS MAY CONTAIN Preamble 1. Preservatives are substances which may be added to cosmetic products for the primary purpose of inhibiting the development
ANNEX VI LIST OF PRESERVATIVES WHICH COSMETIC PRODUCTS MAY CONTAIN Preamble 1. Preservatives are substances which may be added to cosmetic products for the primary purpose of inhibiting the development
Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents
 Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents Sharing expertise around the world As a global manufacturer of cosmetic ingredients and functional fine chemicals, Hannong Chemicals
Rheology Modifier Thickening Emulsifier Viscosity Controlling Agents Sharing expertise around the world As a global manufacturer of cosmetic ingredients and functional fine chemicals, Hannong Chemicals
(12) United States Patent (10) Patent No.: US 6,841,523 B1
 USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
USOO6841523B1 (12) United States Patent (10) Patent No.: US 6,841,523 B1 Holtz (45) Date of Patent: Jan. 11, 2005 (54) NAIL POLISH REMOVER 4,867,800 A 9/1989 Dishart et al. 5,007,969 A 4/1991 Doscher (75)
United States Patent (19) 11 4,344,932 Gordon 45 Aug. 17, 1982
 United States Patent (19) 11 Gordon 45 Aug. 17, 1982 54 NAIL CLEANSER 2,764,168 9/1956 Herz... 132/73 3,1,048 9/1964 Hollab... 424/6 (75) Inventor: Harry W. Gordon, New York, N.Y. 3,384,592 5/1968 Weems...
United States Patent (19) 11 Gordon 45 Aug. 17, 1982 54 NAIL CLEANSER 2,764,168 9/1956 Herz... 132/73 3,1,048 9/1964 Hollab... 424/6 (75) Inventor: Harry W. Gordon, New York, N.Y. 3,384,592 5/1968 Weems...
(12) (10) Patent No.: US 6,971,424 B1. Angevine (45) Date of Patent: Dec. 6, (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi...
 United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
United States Patent USOO6971424B1 (12) (10) Patent No.: Angevine (45) Date of Patent: Dec. 6, 2005 (54) INTERCHANGEABLE HANDBAG 4,112,991 A 9/1978 Barbaresi... 383/13 4.263,951 4/1981 Siegel...... 150/113
(12) United States Patent (10) Patent No.: US 9, B2
 USOO9566228B2 (12) United States Patent (10) Patent No.: US 9,566.228 B2 Jeong (45) Date of Patent: Feb. 14, 2017 (54) BUBBLE TYPE WATERLESS SHAMPOO (2013.01); A61K 8/442 (2013.01); A61K 8/64 COMPOSITION
USOO9566228B2 (12) United States Patent (10) Patent No.: US 9,566.228 B2 Jeong (45) Date of Patent: Feb. 14, 2017 (54) BUBBLE TYPE WATERLESS SHAMPOO (2013.01); A61K 8/442 (2013.01); A61K 8/64 COMPOSITION
(12) United States Patent (10) Patent No.: US 6,894,087 B2
 USOO68987B2 (12) United States Patent (10) Patent No.: Batlaw () Date of Patent: May 17, 2005 (54) TONER COMPOSITIONS FOR BLACK 4,284,729 A 8/1981 Cross et al.... 521/8 GRAVURE INKS 4,383,8 A 5/1983 Iyengar......
USOO68987B2 (12) United States Patent (10) Patent No.: Batlaw () Date of Patent: May 17, 2005 (54) TONER COMPOSITIONS FOR BLACK 4,284,729 A 8/1981 Cross et al.... 521/8 GRAVURE INKS 4,383,8 A 5/1983 Iyengar......
MATERIAL SAFETY DATA SHEET Finished Product.
 MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Nice n Easy Perfect 10 This product is sold as a three component kit:
MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Nice n Easy Perfect 10 This product is sold as a three component kit:
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1. Reynolds et al. (43) Pub. Date: Jan. 15, 2004
 (19) United States US 2004001 0311A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0010311 A1 Reynolds et al. (43) Pub. Date: (54) CUSTOMIZABLE INTEGRATED (21) Appl. No.: 10/196,311 PROSTHETIC
(19) United States US 2004001 0311A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0010311 A1 Reynolds et al. (43) Pub. Date: (54) CUSTOMIZABLE INTEGRATED (21) Appl. No.: 10/196,311 PROSTHETIC
Gafquat 440, 755N, 755N-P, 755N-O and HS-100, HS-100-O polymers Cationic conditioning copolymers
 PRODUCT DATA Consumer Specialties ashland.com NUMBER 4817-1 (Supersedes 4817) Page 1 of 8 Gafquat 440, 755N, 755N-P, 755N-O and HS-100, HS-100-O polymers Cationic conditioning copolymers Introduction Gafquat
PRODUCT DATA Consumer Specialties ashland.com NUMBER 4817-1 (Supersedes 4817) Page 1 of 8 Gafquat 440, 755N, 755N-P, 755N-O and HS-100, HS-100-O polymers Cationic conditioning copolymers Introduction Gafquat
(12) United States Patent
 (12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
(12) United States Patent USOO891 0316B2 (10) Patent No.: US 8,910,316 B2 Albright (45) Date of Patent: Dec. 16, 2014 (54) HEAD COVER 932,968 * 8/1909 Cuddeback... 2,204 2,199.427 A 1/1938 Dohen (76) Inventor:
(12) United States Patent
 US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
US007434336 B2 (12) United States Patent Kosted (10) Patent No.: (45) Date of Patent: US 7434,336 B2 Oct. 14, 2008 (54) FOOTWEAR INCORPORATINGA SELF-ILOCKINGSOCK (76) Inventor: Dale Kosted, 3502 King St.,
AAAAAO Y MAAwa W4WGAAATHIS. July 4, 97. D., P. ESERSEK E Ai. 3,520,31 AAP/ASA ASAA. A77OAweys WWMAW7OAS
 July 4, 97. D., P. ESERSEK E Ai. 3,20,31 COMB WITH FLUID DISTRIBUTION MEANS AND MEANS FOR ATTACHING Filed May 2, 1967 HAIR CARE DEVICE 3 Sheets-Sheet s WWMAW7OAS AAP/ASA ASAA AAAAAO Y MAAwa W4WGAAATHIS
July 4, 97. D., P. ESERSEK E Ai. 3,20,31 COMB WITH FLUID DISTRIBUTION MEANS AND MEANS FOR ATTACHING Filed May 2, 1967 HAIR CARE DEVICE 3 Sheets-Sheet s WWMAW7OAS AAP/ASA ASAA AAAAAO Y MAAwa W4WGAAATHIS
TEGO Carbomer 140 G TEGO Carbomer G
 TEGO 140 G TEGO 141 1 G Convenient granulated viscosity adjusters and builders, emulsion stabilizers Granulated s with numerous advantages: lower dusting, easier to process, higher bulk density First s
TEGO 140 G TEGO 141 1 G Convenient granulated viscosity adjusters and builders, emulsion stabilizers Granulated s with numerous advantages: lower dusting, easier to process, higher bulk density First s
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 2008011 6236A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0116236A1 NICKELS (43) Pub. Date: May 22, 2008 (54) COMBINATION UMBRELLA, SUPPORTAND Publication Classification
(19) United States US 2008011 6236A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0116236A1 NICKELS (43) Pub. Date: May 22, 2008 (54) COMBINATION UMBRELLA, SUPPORTAND Publication Classification
United States Patent (19) Katz
 United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
United States Patent (19) Katz 54 COMBINATION TOY AND GARMENT 76) Inventor: Robert F. Katz, 1401 Manzanita St., Manhattan Beach, Calif. 90266 21 Appl. No.: 593,560 (22) Filed: Mar. 26, 1984 51) Int. Cl....
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
(19) United States US 2003O155389A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0155389 A1 Swartzentruber (43) Pub. Date: Aug. 21, 2003 (54) SLAPON WATCH (52) U.S. Cl.... 224/164 (76) Inventor:
Chemistry is the scientific study of matter and the physical and chemical changes of matter.
 E-HAIR COLLEGE 1. Read Chapter in Salon Fundamental textbook. 2. Complete study guide. 3. Read these additional notes. 4. For review go to Practice online and review quizzes, puzzles. 5. Study and complete
E-HAIR COLLEGE 1. Read Chapter in Salon Fundamental textbook. 2. Complete study guide. 3. Read these additional notes. 4. For review go to Practice online and review quizzes, puzzles. 5. Study and complete
(12) United States Patent (10) Patent No.: US 8,108,948 B2
 USOO8108948B2 (12) United States Patent (10) Patent No.: US 8,108,948 B2 B00s (45) Date of Patent: *Feb. 7, 2012 (54) METHOD AND APPARATUS FOR KEEPING A 3,161,932 A 12/1964 Russell SHIRT COLLAR ALIGNED
USOO8108948B2 (12) United States Patent (10) Patent No.: US 8,108,948 B2 B00s (45) Date of Patent: *Feb. 7, 2012 (54) METHOD AND APPARATUS FOR KEEPING A 3,161,932 A 12/1964 Russell SHIRT COLLAR ALIGNED
12) United States Patent 10) Patent No.: US 6,433,024 B1
 USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
USOO6433024B1 12) United States Patent 10) Patent No.: 9 9 Popp et al. () Date of Patent: Aug. 13, 2002 (54) TOPICAL ANTI-ACNE COMPOSITION OTHER PUBLICATIONS (76) Inventors: Karl F. Popp, 1775 Duck Pond
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
(19) United States US 20110265810A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0265810 A1 PELUS et al. (43) Pub. Date: (54) HAIR CARE COMPOSITIONS Publication Classification (51) Int. Cl.
CREAM NO AMMONIA NO PPD NO PARABEN NO RESORCINOL NO SILICONE NO METAL NO MINERAL OILS NO ARTIFICIAL PERFUME
 CREAM NO AMMONIA NO PPD NO PARABEN NO RESORCINOL NO SILICONE NO METAL NO MINERAL OILS NO ARTIFICIAL PERFUME Indications Permanent colour without PPD, with prodigious oils, validated by clinical trials.
CREAM NO AMMONIA NO PPD NO PARABEN NO RESORCINOL NO SILICONE NO METAL NO MINERAL OILS NO ARTIFICIAL PERFUME Indications Permanent colour without PPD, with prodigious oils, validated by clinical trials.
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 2016.0143424A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0143424 A1 STEPHENS et al. (43) Pub. Date: May 26, 2016 (54) WEARABLE ELASTIC BAND WITH Publication Classification
(19) United States US 2016.0143424A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0143424 A1 STEPHENS et al. (43) Pub. Date: May 26, 2016 (54) WEARABLE ELASTIC BAND WITH Publication Classification
United States Patent (19) Humbrecht
 United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
United States Patent (19) Humbrecht 54) PULL DOWN SKI MASK 76) Inventor: Phyllis A. Humbrecht, 301 Audubon Trail. Fort Wayne. Ind. 46825 (21 Appl. No.: 679,999 22 Filed: Jul. 15, 1996 (51) Int. Cl....
United States Patent (19) Schunter
 United States Patent (19) Schunter 11 45 US005699555A Patent Number: Date of Patent: Dec. 23, 1997 54 CHILD'S WAISTBELTAND LEASH FOR PROTECTIONAGAINSTABDUCTION OF A CHLD 76 Inventor: Christine K. Schunter,
United States Patent (19) Schunter 11 45 US005699555A Patent Number: Date of Patent: Dec. 23, 1997 54 CHILD'S WAISTBELTAND LEASH FOR PROTECTIONAGAINSTABDUCTION OF A CHLD 76 Inventor: Christine K. Schunter,
(*) Notice: Slity sight 58 $5 E. G.
 USOO629.7208B1 (12) United States Patent (10) Patent No.: US 6,297,208 B1 Crist () Date of Patent: Oct. 2, 2001 (54) RUST STAIN REMOVAL FORMULA 4,891,0 1/1990 Gross et al.. 4,963,233 * 10/1990 Mathew...
USOO629.7208B1 (12) United States Patent (10) Patent No.: US 6,297,208 B1 Crist () Date of Patent: Oct. 2, 2001 (54) RUST STAIN REMOVAL FORMULA 4,891,0 1/1990 Gross et al.. 4,963,233 * 10/1990 Mathew...
(12) United States Patent (10) Patent No.: US 7,585,200 B1
 US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
US00758520OB1 (12) United States Patent (10) Patent No.: McLaren (45) Date of Patent: Sep. 8, 2009 (54) POCKET BRA INSERT 817,020 A * 4/1906 Thompson... 450/54 1984,253 A * 12/1934 Cox...... 604,346 (76)
(12) United States Patent (10) Patent No.: US 6,413,305 B1
 USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
USOO6413305B1 (12) United States Patent (10) Patent No.: Mehta et al. (45) Date of Patent: Jul. 2, 2002 (54) THERMOCHROMIC INK COMPOSITION 6,139,779 A * 10/2000 Small et al.... 2/583 (75) Inventors: Rajendra
PO Box 5411 Arlington, TX SF A-348
 SF A-348 PRODUCT DESCRIPTION SF A-348 organosilicone is a proprietary copolymer that represents a class of aminosilicone polyalkyleneoxide copolymers for hair conditioning. Conventional organomodified
SF A-348 PRODUCT DESCRIPTION SF A-348 organosilicone is a proprietary copolymer that represents a class of aminosilicone polyalkyleneoxide copolymers for hair conditioning. Conventional organomodified
IIII. United States Patent (19) McCausland. cover removably attached to the outer edge of said
 United States Patent (19) McCausland 54 FACE SHIELD (76) Inventor: Mary L. McCausland, 16629 Lescot Ter. Rockville, Md. 20853 21) Appl. No.: 658,520 22 Filed: Jun. 4, 1996 (51) Int. Cl.... A61F 9/00; A61F
United States Patent (19) McCausland 54 FACE SHIELD (76) Inventor: Mary L. McCausland, 16629 Lescot Ter. Rockville, Md. 20853 21) Appl. No.: 658,520 22 Filed: Jun. 4, 1996 (51) Int. Cl.... A61F 9/00; A61F
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 US 20060231567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0231567 A1 Perrone (43) Pub. Date: Oct. 19, 2006 (54) CON OPERATED SUNTAN LOTION SPRAY Publication Classification
US 20060231567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0231567 A1 Perrone (43) Pub. Date: Oct. 19, 2006 (54) CON OPERATED SUNTAN LOTION SPRAY Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014009.4718A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0094718A1 Feldman (43) Pub. Date: (54) SYSTEMAND METHOD FORTATTOO (52) U.S. Cl. REMOVAL USPC... 601A2 (71)
(19) United States US 2014009.4718A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0094718A1 Feldman (43) Pub. Date: (54) SYSTEMAND METHOD FORTATTOO (52) U.S. Cl. REMOVAL USPC... 601A2 (71)
(12) United States Patent (10) Patent No.: US 8,770,209 B2
 US008770209B2 (12) United States Patent (10) Patent No.: Kim et al. (45) Date of Patent: Jul. 8, 2014 (54) COLOR HIGHILIGHTING COSMETICS (52) U.S. Cl. CONTAINER INCLUDING ADETACHABLE USPC... 132/297: 132/318;
US008770209B2 (12) United States Patent (10) Patent No.: Kim et al. (45) Date of Patent: Jul. 8, 2014 (54) COLOR HIGHILIGHTING COSMETICS (52) U.S. Cl. CONTAINER INCLUDING ADETACHABLE USPC... 132/297: 132/318;
INTRODUCING PREMIUM BODY CARE
 INTRODUCING PREMIUM BODY CARE WFM Body Care Quality Standards Already the HIGHEST IN THE INDUSTRY. We CAREFULLY EVALUATE each and every product we sell. All personal care products must meet our CURRENT
INTRODUCING PREMIUM BODY CARE WFM Body Care Quality Standards Already the HIGHEST IN THE INDUSTRY. We CAREFULLY EVALUATE each and every product we sell. All personal care products must meet our CURRENT
Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries.
 Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. I. Preservation Naturally-derived product Broad spectrum protection Globally accepted II. Moisturization
Product Information Geogard ULTRA Multifunctional specialty additive for cosmetics and toiletries. I. Preservation Naturally-derived product Broad spectrum protection Globally accepted II. Moisturization
United States Patent (19)
 USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
USOO5958984A 11 Patent Number: Devillez () Date of Patent: *Sep. 28, 1999 United States Patent (19) 54 METHOD AND FOR SKIN 4,363,8 12/1982 Yu et al.... 424/274 TREATMENT 4,431,631 2/1984 Clipper et al..
III. United States Patent 19 Jordan 5,389,129. Feb. 14, ). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee:
 United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
United States Patent 19 Jordan 54). WAXPOLISH COMPOSITION 75 Inventor: Martin P. Jordan, Orpington, 73) Assignee: England Berwind Pharmaceutical Services, Inc., West Point, Pa. 21 Appl. No.: 889,775 22
MATERIAL SAFETY DATA SHEET Finished Product.
 MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Natural Instincts Brass Free This product is sold as a four component
MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Natural Instincts Brass Free This product is sold as a four component
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. o.: US 2015/0068548 A1 Hawkes et al. US 2015.0068548A1 (43) Pub. Date: (54) HAIR TREATMET METHODS (71) Applicant: Perachem Limited, Leeds
(19) United States (12) Patent Application Publication (10) Pub. o.: US 2015/0068548 A1 Hawkes et al. US 2015.0068548A1 (43) Pub. Date: (54) HAIR TREATMET METHODS (71) Applicant: Perachem Limited, Leeds
United States Patent (19) Frankel
 United States Patent (19) Frankel 11 Patent Number: 45 Date of Patent: Jan. 27, 1987 (54) SWEAT COLLECTING HEADBAND 76) Inventor: Alfred R. Frankel, 403 Gulf Way - Apt. 701, St. Petersburg, Fla. 33706
United States Patent (19) Frankel 11 Patent Number: 45 Date of Patent: Jan. 27, 1987 (54) SWEAT COLLECTING HEADBAND 76) Inventor: Alfred R. Frankel, 403 Gulf Way - Apt. 701, St. Petersburg, Fla. 33706
Represented by: Integrity Ingredients Corporation
 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 AMPHOTERIC
Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 Represented by: Integrity Ingredients Corporation info@integrityingredientscorp.com (310) 782-0282 AMPHOTERIC
IN THIS ISSUE NEW ADVANCES LOOKING FORWARD
 NEW AT CLASSIC IN THIS ISSUE SURFACTANT UPDATE The quest continues for mild and natural Page 2 GOT QUAT? simplifying the conditioner picture regarding cationics available at Classic Page 3 NATURAL PRESERVATIVES
NEW AT CLASSIC IN THIS ISSUE SURFACTANT UPDATE The quest continues for mild and natural Page 2 GOT QUAT? simplifying the conditioner picture regarding cationics available at Classic Page 3 NATURAL PRESERVATIVES
United States Patent (19)
 United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
United States Patent (19) USOO5890637A 11 Patent Number: 5,890,637 Furneaux (45) Date of Patent: Apr. 6, 1999 54 PET LEASH MULTI-PURPOSE UTILITY BAG Attorney, Agent, or Firm Antony C. Edwards 76 Inventor:
Please scroll down to view each MSDS.
 This brand has three (3) Material Safety Data Sheets (MSDS) for its ingredients. MSDS are presented in the following order: Revlon Colorist Developer Revlon Colorist Colorant Revlon Colorist Hair Glaze
This brand has three (3) Material Safety Data Sheets (MSDS) for its ingredients. MSDS are presented in the following order: Revlon Colorist Developer Revlon Colorist Colorant Revlon Colorist Hair Glaze
Essential Elements of Rheology Control
 Essential Elements of Rheology Control Richard Giles AkzoNobel Technical Service and Development Manager Europe, Middle East, Africa and India In-cosmetics Barcelona 2015 Outline Today s trends BALANCE
Essential Elements of Rheology Control Richard Giles AkzoNobel Technical Service and Development Manager Europe, Middle East, Africa and India In-cosmetics Barcelona 2015 Outline Today s trends BALANCE
Baltic info campaign on hazardous substances (BaltInfoHaz) - the first international LIFE+ Information and Campaign project in the Baltic States
 THINK BEFORE YOU BUY WWW.THINKBEFORE.EU Baltic info campaign on hazardous substances (BaltInfoHaz) - the first international LIFE+ Information and Campaign project in the Baltic States Think before you
THINK BEFORE YOU BUY WWW.THINKBEFORE.EU Baltic info campaign on hazardous substances (BaltInfoHaz) - the first international LIFE+ Information and Campaign project in the Baltic States Think before you
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O180231A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180231 A1 SCHMENGER et al. (43) Pub. Date: Jul.19, 2012 (54) HAIR COLORING COMPOSITIONS WITH A Publication
(19) United States US 2012O180231A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0180231 A1 SCHMENGER et al. (43) Pub. Date: Jul.19, 2012 (54) HAIR COLORING COMPOSITIONS WITH A Publication
(12) United States Patent (10) Patent No.: US 7,188,625 B2
 US007188625B2 (12) United States Patent (10) Patent No.: US 7,188,625 B2 Durette (45) Date of Patent: Mar. 13, 2007 (54) OCULAR SURGICAL PROTECTIVE SHIELD 4,024.405 A * 5/1977 Szot... 250,516.1 5,390,373
US007188625B2 (12) United States Patent (10) Patent No.: US 7,188,625 B2 Durette (45) Date of Patent: Mar. 13, 2007 (54) OCULAR SURGICAL PROTECTIVE SHIELD 4,024.405 A * 5/1977 Szot... 250,516.1 5,390,373
TEPZZ 6Z69 ZA_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (51) Int Cl.: A61M 39/16 ( )
 (19) TEPZZ 6Z69 ZA_T (11) EP 2 606 930 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.06.2013 Bulletin 2013/26 (51) Int Cl.: A61M 39/16 (2006.01) (21) Application number: 12275026.8 (22)
(19) TEPZZ 6Z69 ZA_T (11) EP 2 606 930 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.06.2013 Bulletin 2013/26 (51) Int Cl.: A61M 39/16 (2006.01) (21) Application number: 12275026.8 (22)
United States Patent (19) 11) 4,197,316
 United States Patent (19) 11) 4,197,316 Yu et al. (45) Apr. 8, 1980 54) TREATMENT OF DRY SKIN 4,021,572 5/1977 Scott et al.... 424/317 76 Inventors: Ruey J. Yu, 4400 Dexter St., OTHER PUBLICATIONS Philadelphia,
United States Patent (19) 11) 4,197,316 Yu et al. (45) Apr. 8, 1980 54) TREATMENT OF DRY SKIN 4,021,572 5/1977 Scott et al.... 424/317 76 Inventors: Ruey J. Yu, 4400 Dexter St., OTHER PUBLICATIONS Philadelphia,
INCI Name: Cyclopentasiloxane (and) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (and) PEG/PPG-20/23 Dimethicone
 Technical Data Sheet Velvesil Plus Velvesil* Plus Gel INCI Name: Cyclopentasiloxane (and) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (and) PEG/PPG-20/23 Dimethicone Product Description Velvesil Plus
Technical Data Sheet Velvesil Plus Velvesil* Plus Gel INCI Name: Cyclopentasiloxane (and) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (and) PEG/PPG-20/23 Dimethicone Product Description Velvesil Plus
American Cleaning Institute Development of Exposure Assessments Glossary of Functional Classes
 American Cleaning Institute Development of Exposure Assessments Glossary of Functional Classes Abrasive: Abrasive ingredients are materials that are used to polish, buff, or scour away soils such as dirt
American Cleaning Institute Development of Exposure Assessments Glossary of Functional Classes Abrasive: Abrasive ingredients are materials that are used to polish, buff, or scour away soils such as dirt
USOO A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999
 USOO5996780A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999 54 COSMETIC APPARATUS 3.513,830 5/1970 Kalayjian... 128/2 3,640,268 2/1972 Davis...... 128/2
USOO5996780A United States Patent (19) 11 Patent Number: 5,996,780 Gurrera (45) Date of Patent: Dec. 7, 1999 54 COSMETIC APPARATUS 3.513,830 5/1970 Kalayjian... 128/2 3,640,268 2/1972 Davis...... 128/2
United States Patent (19) L0da
 United States Patent (19) L0da USOO5858179A 11 Patent Number: 5,858,179 (45) Date of Patent: Jan. 12, 1999 54 METHOD OF TREATING KERATINC FIBERS SUCH ASA MAMMALIAN HAIR WITH A COMBINATION OF CHEMICALS
United States Patent (19) L0da USOO5858179A 11 Patent Number: 5,858,179 (45) Date of Patent: Jan. 12, 1999 54 METHOD OF TREATING KERATINC FIBERS SUCH ASA MAMMALIAN HAIR WITH A COMBINATION OF CHEMICALS
Enhancing Shine in Hair Shineblend Max. Dr. Tony Gough
 Enhancing Shine in Hair Shineblend Max Dr. Tony Gough Consumers desire greater shine There is a growing demand by consumers for healthier looking hair and consumer studies frequently show that hair shine
Enhancing Shine in Hair Shineblend Max Dr. Tony Gough Consumers desire greater shine There is a growing demand by consumers for healthier looking hair and consumer studies frequently show that hair shine
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 2016O152831A1 (12) Patent Application Publication (10) Pub. o.: US 2016/0152831 A1 AKBA et al. (43) Pub. Date: (54) YELLOW COLORAT FOR HAIR DYEIG, C07D 209/.4 (2006.01) COMPOSITIO
(19) United States US 2016O152831A1 (12) Patent Application Publication (10) Pub. o.: US 2016/0152831 A1 AKBA et al. (43) Pub. Date: (54) YELLOW COLORAT FOR HAIR DYEIG, C07D 209/.4 (2006.01) COMPOSITIO
MATERIAL SAFETY DATA SHEET Finished Product.
 MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Koleston Perfect Permanent Creme Haircolor Shades 4 (98797933), 5 (98808029),
MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Koleston Perfect Permanent Creme Haircolor Shades 4 (98797933), 5 (98808029),
Chemical Name : Copolymer of diallyl dimethyl ammonium chloride& acrylamaide.
 HAJ PQ 7 HAJ PQ 7 is a high quality, high purity active ingredient for cosmetics, specifically for hair care & skin care products. CFTA(INCI) Designation: Polyquaternium 7 Chemical Name : Copolymer of
HAJ PQ 7 HAJ PQ 7 is a high quality, high purity active ingredient for cosmetics, specifically for hair care & skin care products. CFTA(INCI) Designation: Polyquaternium 7 Chemical Name : Copolymer of
United States Patent Office
 United States Patent ffice 3,178,371 LPSTICK REMVING METHDS Robert M. Friedenberg, Storrs, Conn., assignor of one half to David L. Berman, West Hartford, Conn. No Drawing. Filed ct. 31, 19, Ser. No. 65,965
United States Patent ffice 3,178,371 LPSTICK REMVING METHDS Robert M. Friedenberg, Storrs, Conn., assignor of one half to David L. Berman, West Hartford, Conn. No Drawing. Filed ct. 31, 19, Ser. No. 65,965
Silsoft* A+ Technical Data Sheet. Silsoft* A+ conditioning agent
 Technical Data Sheet Silsoft* A+ Silsoft* A+ conditioning agent Description Silsoft A+ conditioning agent can help provide excellent conditioning to damaged hair. Silsoft A+ conditioning agent is a surfactant-free
Technical Data Sheet Silsoft* A+ Silsoft* A+ conditioning agent Description Silsoft A+ conditioning agent can help provide excellent conditioning to damaged hair. Silsoft A+ conditioning agent is a surfactant-free
