INSTRUCTIONS FOR USE. English
|
|
|
- Maximillian Dalton
- 5 years ago
- Views:
Transcription
1 INSTRUCTIONS FOR USE English INTENDED USE HepaSphere Microspheres are indicated for use in embolization of blood vessels with or without delivery of doxorubicin HCl for therapeutic or preoperative purposes in the following procedures: Embolization of hepatocellular carcinoma Embolization of metastases to the liver. HepaSphere Microspheres loaded with irinotecan are indicated for use in: Embolization of metastatic colorectal cancer (mcrc) to the liver. DESCRIPTION HepaSphere Microspheres are part of a family of embolic agents based on proprietary technologies. They are designed for controlled, targeted embolization. The HepaSphere Microspheres can be loaded with doxorubicin HCl or irinotecan, and are able to release the drug locally at the embolization site. HepaSphere Microspheres are biocompatible, hydrophilic, non-resorbable, expandable, and conformable microspheres. HepaSphere Microspheres swell upon exposure to aqueous solutions. They are available in a range of sizes. Dry(μm) DEVICE PACKAGING HepaSphere Microspheres are contained in a sterile, 10 ml vial, with a crimped cap, packaged in a sealed pouch. Contents: 25 mg or 50 mg of dry HepaSphere Microspheres per vial to be reconstituted before use. CONTRAINDICATIONS Patients intolerant to vascular occlusion procedures Vascular anatomy or blood flow precluding correct catheter placement or embolic injection Presence or suspicion of vasospasm Presence or likely onset of haemorrhage Presence of severe atheromatous disease Presence of collateral vessel pathways potentially endangering normal territories during embolization High flow arteriovenous shunts or fistulae with luminal diameter greater than the selected size of HepaSphere Microspheres Vascular resistance peripheral to the feeding arteries precluding passage of HepaSphere Microspheres into the lesion Do not use in pulmonary vasculature, coronary and central nervous system vasculature Known sensitivity to poly vinyl alcohol-co-sodium acrylate WARNINGS HepaSphere Microspheres size must be chosen after consideration of the arteriovenous angiographic appearance. HepaSphere Microspheres size should be selected both to be appropriate for the size of the vessel feeding the target and to prevent passage from artery to vein. Some of the HepaSphere Microspheres may be slightly outside of the range, so the physician should be sure to carefully select the size of HepaSphere Microspheres according to the size of the target vessels at the desired level of occlusion in the vasculature and after consideration of the arteriovenous angiographic appearance. Because of the significant complications of untargeted embolization, extreme caution should be used for any procedures involving the extracranial circulation encompassing the head and neck, and the physician should carefully weigh the potential benefits of using embolization against the risks and potential complications of the procedure. These complications can include blindness, hearing loss, loss of smell, paralysis, and death. Serious radiation induced skin injury may occur to the patient due to long periods of fluoroscopic exposure, large patient, angled x-ray projections and multiple image recording runs or radiographs. Refer to your facility s clinical protocol to ensure the proper radiation dose is applied for each specific type of procedure performed. Onset of radiation injury to the patient may be delayed. Patients should be counselled on potential radiation effects, what to look for and whom to contact if symptoms occur. HepaSphere Microspheres MUST NOT be reconstituted in sterile water for injection. Reconstitution in sterile _001_hepasphere_irinotecan.indd 1
2 water results in extensive swelling that renders the injection of HepaSphere Microspheres very difficult or may prevent injection. Do not reconstitute HepaSphere Microspheres with Lipiodol / Ethiodol. Pay careful attention for signs of untargeted embolization. During injection carefully monitor patient vital signs to include SaO 2 (e.g. hypoxia, CNS changes). Consider terminating the procedure, investigating for possible shunting, or increasing Microspheres size if any signs of untargeted embolization occur or patient symptoms develop. Consider upsizing the Microspheres if angiographic evidence of embolization does not quickly appear evident during injection of the Microspheres. Warnings about use of small microspheres: Careful consideration should be given whenever use is contemplated of embolic agents that are smaller in diameter than the resolution capability of your imaging equipment. The presence of arteriovenous anastomoses, branch vessels leading away from the target area or emergent vessels not evident prior to embolization can lead to untargeted embolization and severe complications. Microspheres smaller than 100 microns are more likely to terminate circulation to distal tissue. Greater potential of ischemic injury results from use of smaller sized microspheres and consideration must be given to the consequence of this injury prior to embolization. The potential consequences include swelling, necrosis, paralysis, abscess and/or stronger post-embolization syndrome. Post embolization swelling may result in ischemia to tissue adjacent to target area. Care must be given to avoid ischemia of intolerant, non targeted tissue such as nervous tissue. PRECAUTIONS HepaSphere Microspheres must only be used by physicians trained in vascular embolization procedures. The size and quantity of microspheres must be carefully selected according to the lesion to be treated and the potential presence of shunts. Only the physician can decide the most appropriate time to stop the injection of HepaSphere Microspheres. Do not use if the vial, cap, or pouch appear damaged. For single patient use only - Contents supplied sterile - Never reuse, reprocess, or resterilize the contents of a vial that has been opened. Reusing, reprocessing or resterilizing may compromise the structural integrity of the device and or lead to device failure, which in turn may result in patient injury, illness or death. Reusing, reprocessing or resterilizing may also create a risk of contamination of the device and or cause patient infection or cross infection including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness or death of the patient. All procedures must be performed according to accepted aseptic technique. HepaSphere Microspheres MUST NOT be used in their original dry state. They must be reconstituted before use. HepaSphere Microspheres swell in aqueous solution. The magnitude of swelling depends on the ionic concentration of the solution. The microspheres swell to approximately four times their diameter in 0.9% NaCl aqueous solution and nonionic contrast media, as compared to their initial dry diameter. The magnitude of swelling when loaded with doxorubicin HCl is dependent upon the amount of drug with which the product is loaded. Lyophilized doxorubicin HCl must be reconstituted in NaCl 0.9 % solution. HepaSphere Microspheres undergo a size decrease of about 20% when loaded with doxorubicin HCl, and 30% when loaded with irinotecan compared to the size in pure NaCl 0.9 % aqueous solution. HepaSphere Microspheres are compressible and can be injected easily through microcatheters. However, injection of the HepaSphere Microspheres before they are fully expanded could result in failure to reach the intended embolization target and possible embolization of a larger tissue area. Note: Maximum recommended concentration of doxorubicin HCl is 5mg/ml. Concentrations of doxorubicin HCl above 5mg/ml substantially increase the solution viscosity and make it difficult to handle with HepaSphere Microspheres. Maximum recommended concentration of irinotecan is 20 mg/ml _001_hepasphere_irinotecan.indd 2
3 Patients with known allergies to non-ionic contrast media may require corticosteroids prior to embolization. Additional evaluations or precautions may be necessary in managing periprocedural care for patients with the following conditions: Bleeding diathesis or hypercoagulative state Immunocompromise Note: If loading HepaSphere Microspheres with doxorubicin HCl or irinotecan, refer to the appropriate drug IFU for information concerning contraindications, warnings, precautions, potential complications, dosage, and patient management before use. POTENTIAL COMPLICATIONS Vascular embolization is a high-risk procedure. Complications may occur at any time during or after the procedure, and may include, but are not limited to, the following: Paralysis resulting from untargeted embolization or ischemic injury from adjacent tissue oedema Undesirable reflux or passage of HepaSphere Microspheres into normal arteries adjacent to the targeted lesion or through the lesion into other arteries or arterial beds, such as the internal carotid artery, pulmonary, or coronary circulation Pulmonary embolism due to arteriovenous shunting Ischemia at an undesired location, including ischemic stroke, ischemic infarction (including myocardial infarction), and tissue necrosis Capillary bed occlusion and tissue damage Vasospasm Recanalisation Blindness, hearing loss, and loss of smell Foreign body reactions necessitating medical intervention Infection necessitating medical intervention Complications related to catheterization (e.g. haematoma at the site of entry, clot formation at the tip of the catheter and subsequent dislodgement, and nerve and/or circulatory injuries which may result in leg injury) Allergic reaction to medications (e.g. analgesics) Allergic reaction to non-ionic contrast media or embolic material Vessel or lesion rupture and hemorrhage Death Additional information is found in the Warnings section SWELLING BEHAVIOR HepaSphere Microspheres swell during reconstitution with NaCl 0.9% aqueous solution and non-ionic contrast media. When hydrated in 100% NaCl 0.9% aqueous solution or non-ionic contrast medium, or 50% non-ionic contrast and 50% NaCl 0.9% aqueous solution, HepaSphere Microspheres swell approximately 4 times their original dry diameter in approximately 10 minutes. For example, HepaSphere Microspheres with a diameter of approximately microns in their dry state will expand to approximately microns during reconstitution as recommended below. Because of the inherent variability of the swelling process, some of the HepaSphere Microspheres will be slightly outside of this range after reconstitution, so the physician should be sure to carefully select the size of HepaSphere Microspheres according to the size of the target vessels at the desired level of occlusion in the vasculature and the nature of the aqueous solution. Note: To expand properly HepaSphere Microspheres need to be exposed to a minimum of 10 ml of solution for doxorubicin HCl or saline and a minimum of 5 ml for irinotecan. The magnitude of swelling when loaded with doxorubicin HCl is dependent upon the amount of drug with which the product is loaded. HepaSphere Microspheres undergo a size decrease of about 20% when loaded with doxorubicin HCl compared to the size in pure NaCl 0.9% aqueous solution and about 30 % when loaded with irinotecan. CATHETER COMPATIBILITY HepaSphere Microspheres can be injected with microcatheters with the following specifications: Dry (μm) Approximate Reconstituted Size range (μm) Catheter Size ID(in.) _001_hepasphere_irinotecan.indd 3
4 DIRECTION OF FORCE DIRECTION OF FORCE DIRECTION OF FORCE INSTRUCTIONS HepaSphere Microspheres must be reconstituted with 100% NaCl 0.9% aqueous solution or non-ionic contrast medium, or 50% non-ionic contrast medium and 50% NaCl 0.9% aqueous solution if using without delivery of doxorubicin HCl or irinotecan, or loaded with doxorubicin HCl solution or irinotecan solution before positioning the catheter. Carefully select the size of HepaSphere Microspheres according to the size of the target vessels at the desired level of occlusion in the vasculature and the nature of the aqueous solution. See the description of SWELLING BEHAVIOR. HepaSphere Microspheres may be present outside the vial. Therefore, the vial must be aseptically handled away from the main sterile field. Ensure the compatibility of the HepaSphere Microspheres with the intended size of catheter to be used. See the table above. Inspect the packaging to confirm that it is intact. Remove the vial from the pouch. The external surface of the vial is sterile. To prevent coring the rubber stopper, insert the injection needle as follows: 1. Hold the needle so that the bevel faces upwards and position the tip diagonally to the insertion site. Press the tip against the centre of the insertion site. 2. Apply a gentle force to the needle in the opposite direction to the bevel to ease the needle into the insertion site until the heel section of the needle is no longer visible. Be careful not to scrape off the upper-facing surface of the rubber cap with the heel of the needle tip. 3. Continuing to apply a gentle force to the needle in the opposite direction to the bevel, slowly insert the needle vertically through the rubber cap. 4. After preparation, carefully examine (1) (2) the solution to determine if there are any rubber impurities present. If the solution appears contaminated, do not use it. (3) HEPASPHERE MICROSPHERES CAN BE USED WITH OR WITHOUT LOADING OF DOXORUBICIN HCl OR IRINOTECAN. OPTION 1: PREPARATION FOR EMBOLI- ZATION WITHOUT DRUG (BLAND) The approximate reconstitution time when used without loading of a drug is 10 min. Fill a 10ml syringe with 100% NaCl 0.9% aqueous solution or non-ionic contrast medium (or 50% NaCl 0.9% aqueous solution and 50% contrast). Connect the syringe to a needle of 20 gauge diameter or larger. To ensure proper reconstitution of the HepaSphere Microspheres, grasp the vial horizontally in your fingertips and roll the vial several times. This will transfer the dry contents of the vial to the sidewall. Note: Pull back only the flip-top cap; do not remove the crimp ring or the stopper from the vial. Carefully insert the needle from the syringe through the stopper of the vial. Continue rolling the vial in your fingertips and inject the full amount (10ml) of reconstitution medium into the vial, then place the vial vertically and carefully remove the syringe with the needle attached. Note: The vial is hermetically closed. If aspiration from the syringe into the vial does not automatically occur, then, using caution, manually aspirate air from the vial into the syringe prior to injecting the reconstitution fluid. Proper aspiration and/ or venting techniques, as approved by the healthcare facility, may be used for easier injection of reconstitution medium into vial. If aspiration of air from the vial is performed prior to reconstitution, exercise caution not to remove the spheres from the vial. To ensure a homogeneous reconstitution of the HepaSphere Microspheres, gently invert the vial back and forth so that the liquid contacts the stopper 5-10 times. Note: Vigorous shaking may introduce micro bubbles, which can cause the microspheres to aggregate. Wait a minimum of 10 minutes to allow the HepaSphere Microspheres to reconstitute and expand fully. Use a 30ml syringe and 20 gauge or larger needle to aspirate the contents of the vial. Rotate the vial to a vertical position with the bottom of the vial facing upward. Pull the needle back so _001_hepasphere_irinotecan.indd 4
5 that it is submerged in the liquid but not occluded by the stopper. Gently aspirate the entire contents of the vial into the syringe. Note: If the air was previously aspirated from the vial, gentle injection of air using the syringe prior to aspirating the contents of the vial will ensure an easier aspiration of vial contents into the syringe. If all contents are not withdrawn, introduce an additional volume of air and repeat the aspiration process. It is possible to add an additional amount of non-ionic contrast or NaCl 0.9% aqueous solution into the syringe in order to get a higher dispersion of microspheres. Note: HepaSphere Microspheres reconstituted as described above can be used in the presence of chemotherapeutic agents such as cisplatin, epirubicin, doxorubicin HCl, fluorouracil, irinotecan and mitomycin after hydration. However for drug delivery, HepaSphere Microspheres are only indicated for use with doxorubicin HCl (see below Option 2) or irinotecan (see below Option 3). If microspheres were reconstituted using 100% NaCl 0.9%, non-ionic contrast medium must be added to the syringe containing the HepaSphere Microspheres for visualization under fluoroscopy. If non-ionic contrast medium was used to reconstitute the microspheres, additional non-ionic contrast medium may be added. OPTION 2: PREPARATION FOR EMBOLIZATION LOADED WITH DOXORUBICIN HCl WARNING: Liposomal formulations of doxorubicin HCl are not suitable for loading into HepaSphere Microspheres. As a general guideline the loading of lyophilized doxorubicin HCl solubilized in NaCl 0.9% solution into HepaSphere Microspheres will take one hour. The HepaSphere Microspheres should not be used before they are fully hydrated and expanded. Loading kinetics of pre-solubilized doxorubicin HCI may vary, depending on the concentration and ph of the solution. Choose the appropriate dose of doxorubicin HCl to load into the HepaSphere Microspheres. Note: A maximum dose of doxorubicin HCl 75mg can be loaded into each vial of 25 mg HepaSphere Microspheres. Solubilize the desired dose of lyophilized doxorubicin HCl in 20ml of NaCl 0.9% solution for injection. NEVER USE PURE WATER Note: Maximum recommended concentration of doxorubicin HCl is 5mg/ml. Concentrations of doxorubicin HCl above 5mg/ml substantially increase the solution viscosity and make it difficult to handle with HepaSphere Microspheres. Aspirate the 20ml of doxorubicin HCl solution into two separate 30ml syringes. Each 30ml syringe should contain 10ml of doxorubicin HCl solution. Connect one of the 30ml syringes containing 10ml of the doxorubicin HCl solution to a needle of 20 gauge diameter or larger. To ensure proper reconstitution of the HepaSphere Microspheres, grasp the HepaSphere Microspheres vial horizontally in your fingertips and roll the vial several times. This will transfer the dry contents of the vial to the sidewall. Note: Pull back only the flip-top cap; do not remove the crimp ring or the stopper from the vial. Carefully insert the needle of one of the 30ml syringes containing 10ml of doxorubicin HCl solution through the stopper of the vial. Continue rolling the vial in your fingertips and inject the full 10ml of doxorubicin HCl solution into the vial. Place the HepaSphere Microspheres vial vertically. Carefully remove the syringe with the needle attached, and allow the vial to stand for 10 minutes in order to completely hydrate the spheres. During the 10 minutes hydration period, shake the HepaSphere Microspheres vial several times back and forth so that the liquid contacts the grey stopper. Repeat this process every 2-3 minutes to ensure a homogenous reconstitution of the HepaSphere Microspheres. Note: The vial is hermetically closed. If aspiration from the syringe into the vial does not automatically occur, then, using caution, manually aspirate air from the vial into the syringe prior to injecting the reconstitution fluid. Proper aspiration and/ or venting techniques, as approved by the healthcare facility, may be used for easier injection of reconstitution media into the vial. If aspiration of air from the vial is performed prior to reconstitution, exercise caution not to remove the spheres from the vial. After the 10 minutes hydration period, attach a 20 gauge or larger needle to the second 30ml syringe containing _001_hepasphere_irinotecan.indd 5
6 the remaining 10ml of doxorubicin HCl solution and insert into the HepaSphere Microspheres vial. Aspirate the contents of the HepaSphere Microspheres vial into the 30ml syringe containing the remaining 10 ml of doxorubicin HCl solution. Rotate the vial to a vertical position with the bottom of the vial facing upward. Pull the needle back so that it is submerged in the liquid but not occluded by the stopper. Gently aspirate the entire contents of the vial into the syringe. Prior to removing the needle from the HepaSphere Microspheres vial, while holding the syringe vertically, gently pull the plunger of the syringe down, removing any solution that may be in the hub of the needle. Replace the needle with a syringe cap and invert the syringe back and forth to disperse the contents within the syringe. Wait a minimum of 60 minutes to allow the HepaSphere Microspheres to expand fully and load the doxorubicin HCl. During the 60 minutes, the syringe should be inverted every minutes in order to optimize the drug distribution into the spheres. After 60 minutes, let the syringe stand for the spheres to settle down and purge all supernatant and discard it following facility approved standards. Add a minimum of 20ml of non-ionic contrast medium to the 30ml syringe containing the doxorubicin HCl loaded HepaSphere Microspheres, however larger volume of solution can provide better control during embolization. Gently invert the syringe 2 or 3 times and wait 5 min until solution homogeneity is reached. Before any injection, check the spheres are in suspension, if not, invert the syringe back and forth to disperse contents within the syringe. OPTION 3: PREPARATION FOR EMBOLIZATION LOADED WITH IRINOTECAN HepaSphere Microspheres loaded with irinotecan are only applicable to the 30-60μ and μ sizes. As a general guideline the loading of irinotecan into HepaSphere Microspheres will take 30 minutes. The HepaSphere Microspheres should not be used before they are fully hydrated and expanded. Choose the appropriate dose of irinotecan solution to load into the HepaSphere Microspheres. A maximum dose of 100 mg irinotecan can be loaded in each vial of 25 mg HepaSphere MicroSpheres. Irinotecan solution is typically available in a concentration of 20 mg/ml. Aspirate the irinotecan into a syringe connected to a needle of 20 gauge diameter or larger. To ensure proper reconstitution of the HepaSphere Microspheres, grasp the HepaSphere Microspheres vial horizontally in your fingertips and roll the vial several times. This will transfer the dry contents of the vial to the sidewall. Note: Pull back only the flip-top cap; do not remove the crimp ring or the stopper from the vial. Carefully insert the needle of the syringe containing the irinotecan solution through the stopper of the vial. Continue rolling the vial in your fingertips and inject the irinotecan solution into the vial. Place the HepaSphere Microspheres vial vertically. Carefully remove the syringe with the needle attached, and allow the vial to stand for 30 minutes in order to completely hydrate the spheres. During those 30 minutes, shake the HepaSphere Microspheres vial several times back and forth so that the liquid contacts the grey stopper. Repeat this process every 2-3 minutes to ensure a homogenous reconstitution of the HepaSphere Microspheres. Note: The vial is hermetically closed. If aspiration from the syringe into the vial does not automatically occur, then, using caution, manually aspirate air from the vial into the syringe prior to injecting the reconstitution fluid. Proper aspiration and/or venting techniques, as approved by the healthcare facility, may be used for easier injection of reconstitution media into the vial. If aspiration of air from the vial is performed prior to reconstitution, exercise caution not to remove the spheres from the vial. After the 30 minutes hydration and loading period, attach a 20 gauge or larger needle to an appropriately sized syringe and insert it into the HepaSphere Microspheres vial. Aspirate the contents of the HepaSphere Microspheres vial into the syringe. Rotate the vial to a vertical position with the _001_hepasphere_irinotecan.indd 6
7 bottom of the vial facing upward. Pull the needle back so that it is submerged in the liquid but not occluded by the stopper. Gently aspirate the entire contents of the vial into the syringe. Prior to removing the needle from the HepaSphere Microspheres vial, while holding the syringe vertically, gently pull the plunger of the syringe down, removing any solution that may be in the hub of the needle. Replace the needle with a syringe cap and invert the syringe back and forth to disperse the contents within the syringe. Add an equal volume of non-ionic contrast medium to the syringe containing the irinotecan loaded HepaSphere Microspheres immediately before use. Larger volume of non-ionic contrast media can lead to irinotecan release into the supernatant. Gently invert the syringe 2 or 3 times and wait 5 min until solution homogeneity is reached. Before any injection, check that the microspheres are in suspension. If not, invert the syringe back and forth to disperse contents within the syringe. Do not remove the supernatant. DELIVERY INSTRUCTIONS Carefully evaluate the vascular network associated with the target lesion utilizing high resolution imaging. Note: It is important to determine if any arteriovenous shunts are present before beginning embolization. Using standard techniques, position the delivery catheter within the target vessel and the catheter tip as close as possible to the embolization target. Use an injection syringe no larger than 3ml for the delivery of doxorubicin/ irinotecan/bland loaded HepaSphere Microspheres. Use of a 1ml injection syringe is recommended. Aspirate the HepaSphere Microspheres mixture into the injection syringe. Two methods for embolic aliquot sequestering for injection may be used: Option 1: Connect a 3way-stopcock to the syringe containing the doxorubicin/ irinotecan/bland loaded HepaSphere Microspheres to the infusion micro catheter and use a 1ml syringe for injection through the open port of the 3 way-stopcock. Option 2: Serial aliquots of the doxorubicin/irinotecan/bland loaded HepaSphere Microspheres can be drawn from the syringe into a 1ml injection syringe through a 3 way-stop cock that is not attached to the infusion catheter. The 1ml syringe containing each aliquot can be attached independently to the infusion microcatheter and injected. Invert the syringe back and forth to maintain the homogenous suspension of the HepaSphere Microspheres mixture. Under continuous fluoroscopic guidance, inject the aliquot of HepaSphere Microspheres in a slow, non forceful, pulsatile manner over a time period of approximately 1 minute per ml of microspheres solution. Always inject under free-flow conditions and monitor for reflux. Note: Reflux of embolic spheres can induce immediate ischemia of untargeted tissues and vessels. When stasis in the feeding pedicle occurs while delivering the doxorubicin/ irinotecan/bland loaded HepaSphere Microspheres, wait a minimum of 5 minutes then perform a selective angiogram after the full 5 minutes wait to verify the cessation of antegrade flow. If cessation of antegrade flow has not occurred, continue infusion under fluoroscopic guidance until the desired devascularization is obtained. After the HepaSphere Microsphere infusion is completed, remove the catheter while maintaining gentle aspiration to avoid dislodging any residual HepaSphere Microspheres that may still be in the catheter lumen. Discard the catheter after removal and do not reuse. Discard any open vial or unused HepaSphere Microspheres. CAUTION In the event that the catheter becomes obstructed or significant infusion resistance is encountered during injection, do not attempt to flush the catheter with excessive pressure because reflux of embolic material may occur resulting in untargeted embolization. Remove the catheter while applying gentle aspiration and discard. CONSERVATION AND STORAGE HepaSphere Microspheres must be stored in a dry, dark place in their original vials and packaging. Use by the date indicated on the labeling _001_hepasphere_irinotecan.indd 7
8 When the procedure of reconstitution is completed, store the solution of HepaSphere Microspheres in 2 to 8 C conditions and use within 24 hours, IF not used immediately. Do not store HepaSphere Microspheres after contrast medium has been added. Size of dry products (μm) Colour code (label borders) Orange Yellow Blue Red Quantity of microspheres (mg) Reference V 225 HS V 250 HS V 325 HS V 350 HS V 525 HS V 550 HS V 725 HS V 750 HS _001_hepasphere_irinotecan.indd 8
9 INFORMATION ON PACKAGING Symbol LOT Designation Manufacturer: Name & Address Use by date: year-month-day Batch code Catalog number Do not resterilize Do not use if package is damaged Keep away from sunlight Keep dry Do not re-use Caution: Consult accompanying documents Non-pyrogenic Sterilized using irradiation EC mark logo - Notified body identification: 0459 Size of dry microspheres / Size of hydrated microspheres All serious or life threatening adverse events or deaths associated with use of HepaSphere Microspheres should be reported to the device manufacturer. 0459_ Biosphere Medical, S.A. Parc des Nations - Paris Nord rue de la Belle Etoile Roissy en France France Manufactured for: Merit Medical Systems, Inc West Merit Parkway South Jordan, Utah 84095, U.S.A U.S.A. Customer Service _ _001_hepasphere_irinotecan.indd 9 1/7/16 8:23 AM
English INSTRUCTIONS FOR USE
 English INTENDED USE HepaSphere Microspheres are indicated for use in embolisation of blood vessels with or without delivery of doxorubicin HCI for therapeutic or preoperative purposes in the following
English INTENDED USE HepaSphere Microspheres are indicated for use in embolisation of blood vessels with or without delivery of doxorubicin HCI for therapeutic or preoperative purposes in the following
Loading HepaSphere Microspheres with Lyophilized Doxorubicin
 Loading HepaSphere Microspheres with Lyophilized Doxorubicin 1 A Step-by-Step Reference for Clinicians in 1 vial Supplies 1 x doxorubicin powder vial 1 HepaSphere Microspheres vial 4 x 20G needles or larger
Loading HepaSphere Microspheres with Lyophilized Doxorubicin 1 A Step-by-Step Reference for Clinicians in 1 vial Supplies 1 x doxorubicin powder vial 1 HepaSphere Microspheres vial 4 x 20G needles or larger
Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System. Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe
 Chapter 6 Phlebotomy Procedure 29 Performing A Venipuncture Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe Procedure
Chapter 6 Phlebotomy Procedure 29 Performing A Venipuncture Procedure 30 Collecting A Blood Specimen Using The Vacuum-Tube System Procedure 31 Collecting A Blood Specimen Using A Needle And Syringe Procedure
Migraine Attack Abortive Treatment Medication Overuse Protocol Treatment Refractory Cluster Headache Treatment
 D i h y d r o e r g o t a m i n e ( D H E ) S u b c u t a n e o u s I n j e c t i o n G u i d e Migraine Attack Abortive Treatment Medication Overuse Protocol Treatment Refractory Cluster Headache Treatment
D i h y d r o e r g o t a m i n e ( D H E ) S u b c u t a n e o u s I n j e c t i o n G u i d e Migraine Attack Abortive Treatment Medication Overuse Protocol Treatment Refractory Cluster Headache Treatment
Lenis Needle-free Safety Syringe Device User Manual
 Lenis Needle-free Safety Syringe Device User Manual 1 Table of Contents Welcome.3 Lenis Kit Components.4 Instructions 5-9 Maintenance and Care..10 Troubleshooting. 11 Warranty.12 Precautions 13 Return
Lenis Needle-free Safety Syringe Device User Manual 1 Table of Contents Welcome.3 Lenis Kit Components.4 Instructions 5-9 Maintenance and Care..10 Troubleshooting. 11 Warranty.12 Precautions 13 Return
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
MEDICATION AND INJECTION ADMINISTRATION EDUCATIONAL BOOKLET FOR OVULATION INDUCTION
 MEDICATION AND INJECTION ADMINISTRATION EDUCATIONAL BOOKLET FOR OVULATION INDUCTION Comprehensive Guide to Understanding, Mixing, and Administering Fertility Medications IMPORTANT PHONE NUMBERS: WEEKEND
MEDICATION AND INJECTION ADMINISTRATION EDUCATIONAL BOOKLET FOR OVULATION INDUCTION Comprehensive Guide to Understanding, Mixing, and Administering Fertility Medications IMPORTANT PHONE NUMBERS: WEEKEND
Complete Dermal Integration. Proven Duration.
 Complete Dermal Integration. Proven Duration. Introducing BELOTERO BALANCE Dermal Filler. BELOTERO BALANCE Dermal Filler is uniquely manufactured with CPM Technology to give you precision to treat a wide
Complete Dermal Integration. Proven Duration. Introducing BELOTERO BALANCE Dermal Filler. BELOTERO BALANCE Dermal Filler is uniquely manufactured with CPM Technology to give you precision to treat a wide
Administering ORENCIA (abatacept): Your Step-by-Step Guide
 Administering ORENCIA (abatacept): Your Step-by-Step Guide How to prepare, use and dispose of the abatacept pre-filled syringe or ClickJect pre-filled pen in five steps 427UK1500866-01 Date of preparation:
Administering ORENCIA (abatacept): Your Step-by-Step Guide How to prepare, use and dispose of the abatacept pre-filled syringe or ClickJect pre-filled pen in five steps 427UK1500866-01 Date of preparation:
Appendix L: Accessing/Deaccessing Implanted Central Venous Access Port
 Effective Date: 03/01/2008 Page 1 of 5 Recommendations for Use Insertion Considerations Implanted Port Dressing Access/ Reaccess An implanted port is strongly recommended for patients in whom more than
Effective Date: 03/01/2008 Page 1 of 5 Recommendations for Use Insertion Considerations Implanted Port Dressing Access/ Reaccess An implanted port is strongly recommended for patients in whom more than
Do not take Neulasta if you have had a serious allergic reaction to pegfilgrastim (Neulasta ) or to filgrastim (Neupogen ).
 {SIDE 1 Information} Patient Instructions for Use On-body Injector for Neulasta Description The on-body injector for Neulasta is intended for delivery of Neulasta. The on-body injector is small, for one-time
{SIDE 1 Information} Patient Instructions for Use On-body Injector for Neulasta Description The on-body injector for Neulasta is intended for delivery of Neulasta. The on-body injector is small, for one-time
TRANSGENDER HEALTH Injection Guide
 TRANSGENDER HEALTH Injection Guide 1of the Southern Finger Lakes This information in this booklet has been adapted with permission from a handbook created by Fenway Health. Fenwayhealth.org The instructions
TRANSGENDER HEALTH Injection Guide 1of the Southern Finger Lakes This information in this booklet has been adapted with permission from a handbook created by Fenway Health. Fenwayhealth.org The instructions
Student Performance Guide. Student Performance Guide. Student Performance Guide. Student Performance Guide. LESSON 3-3 Bleeding Time
 LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
LESSON 3-3 Bleeding Time Student Performance Guide LESSON 3-4 Prothrombin Time Student Performance Guide LESSON 3-5 Activated Partial Thromboplastin Time Student Performance Guide LESSON 3-6 Rapid Tests
2 x 1mL. Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy PRINGY-FRANCE
 2 x 1mL Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy 74370 PRINGY-FRANCE Australian Distributor: ALLERGAN Australia Pty Ltd GORDON NSW 2072 New Zealand Distributor: ALLERGAN New
2 x 1mL Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy 74370 PRINGY-FRANCE Australian Distributor: ALLERGAN Australia Pty Ltd GORDON NSW 2072 New Zealand Distributor: ALLERGAN New
How to Give a Subcutaneous (SC) Injection to Your Child
 How to Give a Subcutaneous (SC) Injection to Your Child Supplies: Needles and syringes Alcohol swabs and gauze Vial with the drug solution Sharps container (Health Facts for You #4587) Band-Aids Distraction
How to Give a Subcutaneous (SC) Injection to Your Child Supplies: Needles and syringes Alcohol swabs and gauze Vial with the drug solution Sharps container (Health Facts for You #4587) Band-Aids Distraction
SPECIMEN COLLECTION. A Study Guide
 SPECIMEN COLLECTION A Study Guide Expectations At the UAP Marathon the UAP will be expected to verbalize the process of performing a peripheral blood draw and demonstrating the correct collection of a
SPECIMEN COLLECTION A Study Guide Expectations At the UAP Marathon the UAP will be expected to verbalize the process of performing a peripheral blood draw and demonstrating the correct collection of a
V O L I T E. 2 x 1mL. Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy PRINGY ANNECY-FRANCE
 27 73 70 68 V O L I T E 2 x 1mL Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy PRINGY 74370 ANNECY-FRANCE Australian Distributor: ALLERGAN Australia Pty Ltd GORDON NSW 2072 New Zealand
27 73 70 68 V O L I T E 2 x 1mL Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy PRINGY 74370 ANNECY-FRANCE Australian Distributor: ALLERGAN Australia Pty Ltd GORDON NSW 2072 New Zealand
V O L I F T. 2 x 1mL. Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy PRINGY-FRANCE
 V O L I F T 2 x 1mL Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy 74370 PRINGY-FRANCE Australian Distributor: ALLERGAN Australia Pty Ltd GORDON NSW 2072 New Zealand Distributor:
V O L I F T 2 x 1mL Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy 74370 PRINGY-FRANCE Australian Distributor: ALLERGAN Australia Pty Ltd GORDON NSW 2072 New Zealand Distributor:
Figure A. Figure B To prevent premature activation of the needle safety guard, do not touch the NEEDLE GUARD ACTIVATION CLIPS at any time during use.
 INSTRUCTIONS FOR USE STELARA (stel ar a) (ustekinumab) injection, for subcutaneous use Instructions for injecting STELARA using a prefilled syringe. Read this Instructions for Use before you start using
INSTRUCTIONS FOR USE STELARA (stel ar a) (ustekinumab) injection, for subcutaneous use Instructions for injecting STELARA using a prefilled syringe. Read this Instructions for Use before you start using
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Implantable Venous Port
 Information About Your Child s Procedure Implantable Venous Port Read this form so you understand the procedure and its risks. Please ask questions about anything you do not understand. What is an implantable
Information About Your Child s Procedure Implantable Venous Port Read this form so you understand the procedure and its risks. Please ask questions about anything you do not understand. What is an implantable
Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy PRINGY-FRANCE
 2 x 1mL Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy 74370 PRINGY-FRANCE Australian Distributor: ALLERGAN Australia Pty Ltd GORDON NSW 2072 New Zealand Distributor: ALLERGAN New
2 x 1mL Manufacturer: ALLERGAN Route de Promery Zone Artisanale de Pré-Mairy 74370 PRINGY-FRANCE Australian Distributor: ALLERGAN Australia Pty Ltd GORDON NSW 2072 New Zealand Distributor: ALLERGAN New
Instructions for Use BYDUREON (by-dur-ee-on) Single-Dose Tray (exenatide extended-release) for injectable suspension
 BYDUREON (exenatide extended-release) for injectable suspension 6 Instructions for Use BYDUREON (by-dur-ee-on) Single-Dose Tray (exenatide extended-release) for injectable suspension Before using Bydureon,
BYDUREON (exenatide extended-release) for injectable suspension 6 Instructions for Use BYDUREON (by-dur-ee-on) Single-Dose Tray (exenatide extended-release) for injectable suspension Before using Bydureon,
Topi-CLICK 140 Specifications
 126mm 140 ml Topi-CLICK 140 Specifications Topi-CLICK 140: 140 ml, 0.50 ml per CLICK DESCRIPTION: The NEW Topi-CLICK 140 is an intelligently designed topical dosing device that overcomes the various limitations
126mm 140 ml Topi-CLICK 140 Specifications Topi-CLICK 140: 140 ml, 0.50 ml per CLICK DESCRIPTION: The NEW Topi-CLICK 140 is an intelligently designed topical dosing device that overcomes the various limitations
LACERATION HISTORY TAKING
 SUTURE WORKSHOP :: page 1 LACERATION HISTORY TAKING With any laceration, you must consider several things that will help guide treatment. Always ask exactly how long ago it happened, approximate amount
SUTURE WORKSHOP :: page 1 LACERATION HISTORY TAKING With any laceration, you must consider several things that will help guide treatment. Always ask exactly how long ago it happened, approximate amount
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Pen (75 mg/ml)
 Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Pen (75 mg/ml) Important Information The device is a single-dose disposable pen. It
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Pen (75 mg/ml) Important Information The device is a single-dose disposable pen. It
PENTAMYCETIN is an antibiotic that: Stops the growth of bacteria Kills bacteria
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Read this carefully before you start taking PENTAMYCETIN each time you get a refill. This leaflet is a summary and will
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Read this carefully before you start taking PENTAMYCETIN each time you get a refill. This leaflet is a summary and will
Informed Consent for Dermal Filler
 Informed Consent for Dermal Filler NAME: DATE OF BIRTHG: ADDRESS: CELL PHONE: EMAIL: www.medicaleyecenter.com Please initial all of the following sections confirming that you have read and understand each
Informed Consent for Dermal Filler NAME: DATE OF BIRTHG: ADDRESS: CELL PHONE: EMAIL: www.medicaleyecenter.com Please initial all of the following sections confirming that you have read and understand each
MANAGEMENT OF RADIATION INDUCED SKIN REACTIONS
 Manchester Cancer MANAGEMENT OF RADIATION INDUCED SKIN REACTIONS One of the most common side effects of radiation is acute skin reaction which can range from mild erythema to confluent moist desquamation
Manchester Cancer MANAGEMENT OF RADIATION INDUCED SKIN REACTIONS One of the most common side effects of radiation is acute skin reaction which can range from mild erythema to confluent moist desquamation
Original Date:
 Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Chapter 24. Assisting With Wound Care. Elsevier items and derived items 2014, 2010 by Mosby, an imprint of Elsevier Inc. All rights reserved.
 Chapter 24 Assisting With Wound Care Wound Care A wound is a break in the skin or mucous membrane. The wound is a portal of entry for microbes. Infection is a major threat. Wound care involves: Preventing
Chapter 24 Assisting With Wound Care Wound Care A wound is a break in the skin or mucous membrane. The wound is a portal of entry for microbes. Infection is a major threat. Wound care involves: Preventing
Ease of Use. restore for face and body. refine for more superficial treatments
 Thank you for choosing MD Pen TM, the latest innovation in fractional microdermal needling. MD Pen TM revitalizes the skin by initiating cellular regeneration, and aiding in the absorption of cosmetic
Thank you for choosing MD Pen TM, the latest innovation in fractional microdermal needling. MD Pen TM revitalizes the skin by initiating cellular regeneration, and aiding in the absorption of cosmetic
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
SELECT 1 DAY CONTACT LENS
 PACKAGE INSERT Select 1 Day (somofilcon A) SYMBOLS KEY Soft (hydrophilic) Contact Lenses for Daily Wear Single Use Only The following symbols may appear on the label or carton. SYMBOL LOT! STERILE DEFINITION
PACKAGE INSERT Select 1 Day (somofilcon A) SYMBOLS KEY Soft (hydrophilic) Contact Lenses for Daily Wear Single Use Only The following symbols may appear on the label or carton. SYMBOL LOT! STERILE DEFINITION
A NON-SURGICAL SKIN REJUVENATION TREATMENT
 A NON-SURGICAL SKIN REJUVENATION TREATMENT WHAT IS MESOFILLING? Mesofilling is a new concept of injectable treatment between dermal filling and mesotherapy. Mesotherapy, a safe, effective and established
A NON-SURGICAL SKIN REJUVENATION TREATMENT WHAT IS MESOFILLING? Mesofilling is a new concept of injectable treatment between dermal filling and mesotherapy. Mesotherapy, a safe, effective and established
There are few side-effects in mesotherapy. In most cases, they are minor and reversible:
 Firmzon Inci: Aqua, Carnitine, Cynara Scolimus, Melilot, Socium Hyaluronate, Organic Silicium, Caffein, Troxerutin, Leucine, Valine, Arginine, Glutamine. Properties: Recommended for the treatment of cellulite
Firmzon Inci: Aqua, Carnitine, Cynara Scolimus, Melilot, Socium Hyaluronate, Organic Silicium, Caffein, Troxerutin, Leucine, Valine, Arginine, Glutamine. Properties: Recommended for the treatment of cellulite
Always be on the safe side.
 Instructions for use For SONICflex tips prep crown round, prep crown round A - REF 1.008.6383, 1.008.6384, prep crown plain, prep crown plain A - REF 1.008.6385, 1.008.6386 Always be on the safe side.
Instructions for use For SONICflex tips prep crown round, prep crown round A - REF 1.008.6383, 1.008.6384, prep crown plain, prep crown plain A - REF 1.008.6385, 1.008.6386 Always be on the safe side.
MESOTHERAPY PROTOCOLS
 MESOTHERAPY PROTOCOLS DIRECTORY OF PROTOCOLS 1. Localized Fat Reduction Body Localized Fat Protocol #1 Localized Fat Protocol #2 Localized Fat Protocol #3 Localized Fat Protocol #4 Formula 5 2. Localized
MESOTHERAPY PROTOCOLS DIRECTORY OF PROTOCOLS 1. Localized Fat Reduction Body Localized Fat Protocol #1 Localized Fat Protocol #2 Localized Fat Protocol #3 Localized Fat Protocol #4 Formula 5 2. Localized
NEWS RELEASE. CONTACTS: Investors: Lisa DeFrancesco (862) Media: Mark Marmur (862) Ember Garrett (714)
 NEWS RELEASE CONTACTS: Investors: Lisa DeFrancesco (862) 261-7152 Media: Mark Marmur (862) 261-7558 Ember Garrett (714) 246-3525 JUVÉDERM VOLBELLA XC APPROVED BY U.S. FDA FOR USE IN LIPS AND PERIORAL RHYTIDS
NEWS RELEASE CONTACTS: Investors: Lisa DeFrancesco (862) 261-7152 Media: Mark Marmur (862) 261-7558 Ember Garrett (714) 246-3525 JUVÉDERM VOLBELLA XC APPROVED BY U.S. FDA FOR USE IN LIPS AND PERIORAL RHYTIDS
The Effects of Shear on Neutralized Carbomers in Aqueous Conditions
 The Effects of Shear on Neutralized Carbomers in Aqueous Conditions Lyndel Speedy 18/07/2014 With thanks to Ensign Laboratories 1 Abstract Carbomer is the generic name for a class of high molecular weight
The Effects of Shear on Neutralized Carbomers in Aqueous Conditions Lyndel Speedy 18/07/2014 With thanks to Ensign Laboratories 1 Abstract Carbomer is the generic name for a class of high molecular weight
Instructions for Use. (dulaglutide) injection, for subcutaneous use. 1.5 mg/0.5 ml Single-Dose Pen. once weekly. Unfold and lay flat
 1 BREAK SEAL Instructions for Use TRULICITY (Trū-li-si-tee) BREAK SEAL (dulaglutide) injection, for subcutaneous use 1.5 mg/0.5 ml Single-Dose Pen once weekly Unfold and lay flat Read both sides for full
1 BREAK SEAL Instructions for Use TRULICITY (Trū-li-si-tee) BREAK SEAL (dulaglutide) injection, for subcutaneous use 1.5 mg/0.5 ml Single-Dose Pen once weekly Unfold and lay flat Read both sides for full
Hyaluronic Acid Injections: Incorporating Advanced Microinjection Techniques Into Practice ReachMD Page 1 of 6
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
MARK D. EPSTEIN, M.D. F.A.C.S. Hyaluronic Acid (HA) INJECTION - INFORMATION FOR PATIENTS
 Hyaluronic Acid (HA) INJECTION - INFORMATION FOR PATIENTS INSTRUCTIONS This is an informed-consent document which has been prepared to help you understand hyaluronic acid (Juvederm, Restylane, Belotero)
Hyaluronic Acid (HA) INJECTION - INFORMATION FOR PATIENTS INSTRUCTIONS This is an informed-consent document which has been prepared to help you understand hyaluronic acid (Juvederm, Restylane, Belotero)
EVERYONE WILL NOTICE. No One Will Know.
 THE WORLD S #1 SELLING DERMAL FILLER COLLECTION EVERYONE WILL NOTICE. No One Will Know. Get the natural-looking, long-lasting results you desire. Ask your aesthetic specialist about JUVÉDERM today. Actual
THE WORLD S #1 SELLING DERMAL FILLER COLLECTION EVERYONE WILL NOTICE. No One Will Know. Get the natural-looking, long-lasting results you desire. Ask your aesthetic specialist about JUVÉDERM today. Actual
Press Kit: Primary Messaging
 Press Kit: Primary Messaging The following points outline three key differentiators of Revanesse Versa TM. Using these points as a guideline and basis for content creation will help ensure product claims
Press Kit: Primary Messaging The following points outline three key differentiators of Revanesse Versa TM. Using these points as a guideline and basis for content creation will help ensure product claims
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Informed Consent Hyaluronic Acid Filler Injection
 Informed Consent Hyaluronic Acid Filler Injection INSTRUCTIONS This is an informed-consent document which has been prepared to help inform you about hyaluronic acidbased (non-animal stabilized) tissue
Informed Consent Hyaluronic Acid Filler Injection INSTRUCTIONS This is an informed-consent document which has been prepared to help inform you about hyaluronic acidbased (non-animal stabilized) tissue
SOP: Rodent Identification
 SOP: Rodent Identification These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol
SOP: Rodent Identification These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol
Instructions For Use
 Instructions For Use 1 subcutaneous injection Welcome This guide contains information on how to use Rebif Rebidose, a pre assembled, single use autoinjector that administers one dose of Rebif (interferon
Instructions For Use 1 subcutaneous injection Welcome This guide contains information on how to use Rebif Rebidose, a pre assembled, single use autoinjector that administers one dose of Rebif (interferon
MICRONEEDLING DEVICE (SKIN ROLLER) ENHANCES SKIN S ABSORPTION OF PRODUCTS
 GUNA Clinical Support Doc Dr Jo Serrentino Guna Clinical Support Line 877-486-2383 Clinicalsupport@gunacanada.com GUNA MICRONEEDLING DEVICE APPLICATIONS and TECHNIQUE MICRONEEDLING DEVICE (SKIN ROLLER)
GUNA Clinical Support Doc Dr Jo Serrentino Guna Clinical Support Line 877-486-2383 Clinicalsupport@gunacanada.com GUNA MICRONEEDLING DEVICE APPLICATIONS and TECHNIQUE MICRONEEDLING DEVICE (SKIN ROLLER)
3d-lift. Radically New Approach for Anti-Aging Treatment.
 What is 3d-lift? Embedding therapy needle with absorbable suture (PDO) Injecting several dozen of needles on cheeks one by one. After pulling needles out, the inserted suture stay into the skin. Stimulate
What is 3d-lift? Embedding therapy needle with absorbable suture (PDO) Injecting several dozen of needles on cheeks one by one. After pulling needles out, the inserted suture stay into the skin. Stimulate
Dressings Range Healthcare Ltd
 Dressings Range 365 Healthcare Ltd Unit 1 West Bank Berry Hill Industrial Estate Droitwich Spa Worcestershire WR9 9AX Phone: 01905 778365 Fax: 01905 826110 E mail: info@365healthcare.com Wound Closure
Dressings Range 365 Healthcare Ltd Unit 1 West Bank Berry Hill Industrial Estate Droitwich Spa Worcestershire WR9 9AX Phone: 01905 778365 Fax: 01905 826110 E mail: info@365healthcare.com Wound Closure
Liposomal vitamin C highly concentrated for topical application with SDS system. Restores the physical and mechanical properties of the skin.
 DERMALIFT Facial Care Liposomal vitamin C highly concentrated for topical application with SDS system Restores the physical and mechanical properties of the skin. Intense, fast, profound and lasting e
DERMALIFT Facial Care Liposomal vitamin C highly concentrated for topical application with SDS system Restores the physical and mechanical properties of the skin. Intense, fast, profound and lasting e
Professional Manicure & Pedicure Kit
 ELM-MAN200-0214-01_Layout 1 28/02/2014 15:17 Page 1 Professional Manicure & Pedicure Kit 2 Year Guarantee Instruction Manual By ELM-MAN200- ELM-MAN200-0214-01_Layout 1 28/02/2014 15:17 Page 2 Thank you
ELM-MAN200-0214-01_Layout 1 28/02/2014 15:17 Page 1 Professional Manicure & Pedicure Kit 2 Year Guarantee Instruction Manual By ELM-MAN200- ELM-MAN200-0214-01_Layout 1 28/02/2014 15:17 Page 2 Thank you
BEFORE USING PRODUCT, READ THE FOLLOWING
 Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician or properly licensed practitioner. BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY..
Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician or properly licensed practitioner. BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY..
Revised 1/10. Copyright 2010 St. Jude Children's Research Hospital Page 1 of 5
 Medicines that you give into a muscle are called intramuscular (IM) injections. These injections (shots) are given into areas of the body called injection sites. The nurse will show you the following steps
Medicines that you give into a muscle are called intramuscular (IM) injections. These injections (shots) are given into areas of the body called injection sites. The nurse will show you the following steps
ACETOCAUSTIN 0,5 ml, Cutaneous solution
 PACKAGE LEAFLET: INFORMATION FOR THE USER ACETOCAUSTIN 0,5 ml, Cutaneous solution MONOCHLOROACETIC ACID This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet
PACKAGE LEAFLET: INFORMATION FOR THE USER ACETOCAUSTIN 0,5 ml, Cutaneous solution MONOCHLOROACETIC ACID This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet
Hazardous Substances UHSP/15/HS/05 Schedule 3.5/06. Enhanced Good Chemical Practice for Work with Hydrogen Fluoride and Hydrofluoric Acid
 Health and Safety Policy Hazardous Substances UHSP/15/HS/05 Schedule 3.5/06 HAZARDOUS SUBSTANCES POLICY - CONTROL MEASURES Enhanced Good Chemical Practice for Work with Hydrogen Fluoride and Hydrofluoric
Health and Safety Policy Hazardous Substances UHSP/15/HS/05 Schedule 3.5/06 HAZARDOUS SUBSTANCES POLICY - CONTROL MEASURES Enhanced Good Chemical Practice for Work with Hydrogen Fluoride and Hydrofluoric
Procedure 19 Changing A Clean Dressing. Procedure 20 Applying A Bandage. Procedure 21 Applying A Sterile Dressing
 Chapter 5 Wound Care Procedure 19 Changing A Clean Dressing Procedure 20 Applying A Bandage Procedure 21 Applying A Sterile Dressing Procedure 22 Applying A Dressing Around A Drain Procedure 23 Changing
Chapter 5 Wound Care Procedure 19 Changing A Clean Dressing Procedure 20 Applying A Bandage Procedure 21 Applying A Sterile Dressing Procedure 22 Applying A Dressing Around A Drain Procedure 23 Changing
GETTING COMFORTABLE WITH TAKING STELARA
 GETTING COMFORTABLE WITH TAKING STELARA Your easy-to-follow overview of self-injection This guide is a supplement to the full Instructions for Use. This guide is not intended to replace those instructions.
GETTING COMFORTABLE WITH TAKING STELARA Your easy-to-follow overview of self-injection This guide is a supplement to the full Instructions for Use. This guide is not intended to replace those instructions.
Patients should be given information about skin reactions and self-care strategies. A recent UK survey found that:
 Summary of Interventions for Acute Radiotherapy-Induced Skin Reactions in Cancer Patients: A Clinical Guideline recommended for use by The Society and; College of Radiographers Responsible person: Rachel
Summary of Interventions for Acute Radiotherapy-Induced Skin Reactions in Cancer Patients: A Clinical Guideline recommended for use by The Society and; College of Radiographers Responsible person: Rachel
I.V. INJECTION ARM P50/1 ( ) USER MANUAL
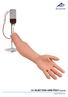 I.V. INJECTION ARM P50/1 (1021418) USER MANUAL The I.V. Injection Arm P50/1 is an educational system developed to assist a certified instructor. It is not a substitute for a comprehensive understanding
I.V. INJECTION ARM P50/1 (1021418) USER MANUAL The I.V. Injection Arm P50/1 is an educational system developed to assist a certified instructor. It is not a substitute for a comprehensive understanding
WHERE HEALING HAPPENS TWO-STEP HOSPITAL-GRADE SYSTEM RADIATION SKIN CARE
 AT HOME WHERE HEALING HAPPENS TWO-STEP HOSPITAL-GRADE SYSTEM RADIATION SKIN CARE Cleanses, moisturizes and protects red, irritated skin Helps protect against redness, drying and peeling Radiation Dermatitis
AT HOME WHERE HEALING HAPPENS TWO-STEP HOSPITAL-GRADE SYSTEM RADIATION SKIN CARE Cleanses, moisturizes and protects red, irritated skin Helps protect against redness, drying and peeling Radiation Dermatitis
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
Medications and Medication Safety
 Specialty Pharmacy Welcome Packet Medications and Medication Safety Address: 9605 57th Avenue Corona, NY 11368 844-334-9615 toll-free phone 844.941.4111 toll-free fax Website http://www.21centurypharmacy.com/
Specialty Pharmacy Welcome Packet Medications and Medication Safety Address: 9605 57th Avenue Corona, NY 11368 844-334-9615 toll-free phone 844.941.4111 toll-free fax Website http://www.21centurypharmacy.com/
STELARA INJECTION. What is in this leaflet. Before you use STELARA. What STELARA is used for. Consumer Medicine Information
 STELARA INJECTION Ustekinumab (rmc) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about STELARA (pronounced stel-ahr-uh). It does not contain all the
STELARA INJECTION Ustekinumab (rmc) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about STELARA (pronounced stel-ahr-uh). It does not contain all the
Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid
 Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid Purpose: This Safe Method of Use applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs),
Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid Purpose: This Safe Method of Use applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs),
BAXTER ANNOUNCES U.S. FDA APPROVAL AND COMMERCIAL LAUNCH OF READY-TO-USE CLINDAMYCIN INJECTION IN SALINE
 FOR IMMEDIATE RELEASE Media Contact Eric Tatro, (224) 948-5353 media@baxter.com Investor Contact Clare Trachtman, (224) 948-3085 BAXTER ANNOUNCES U.S. FDA APPROVAL AND COMMERCIAL LAUNCH OF READY-TO-USE
FOR IMMEDIATE RELEASE Media Contact Eric Tatro, (224) 948-5353 media@baxter.com Investor Contact Clare Trachtman, (224) 948-3085 BAXTER ANNOUNCES U.S. FDA APPROVAL AND COMMERCIAL LAUNCH OF READY-TO-USE
I. DEVICE DESCRIPTION
 Derma Veil INSTRUCTIONS FOR USE BEFORE USING DERMA VEIL PLEASE READ THE FOLLOW ING INFO RMATION. IF YOU HAVE ANY QUESTIONS REGARDING THESE GUIDELINES CONTACT YOUR LOCAL DISTRIBUTOR OR MEDINTER, LTD. AT:
Derma Veil INSTRUCTIONS FOR USE BEFORE USING DERMA VEIL PLEASE READ THE FOLLOW ING INFO RMATION. IF YOU HAVE ANY QUESTIONS REGARDING THESE GUIDELINES CONTACT YOUR LOCAL DISTRIBUTOR OR MEDINTER, LTD. AT:
BlephEx...A Revolutionary New Treatment for Blepharitis. Owner s Manual
 BlephEx...A Revolutionary New Treatment for Blepharitis Owner s Manual Table of Contents Warnings...2 Intended Use...2 Instructions for Use...3 Cleaning Instructions...8 Maintenance Details...8 Safety
BlephEx...A Revolutionary New Treatment for Blepharitis Owner s Manual Table of Contents Warnings...2 Intended Use...2 Instructions for Use...3 Cleaning Instructions...8 Maintenance Details...8 Safety
(Injection of collagen, hyaluronic acid or other filler materials) INFORMED CONSENT FOR DERMAL FILLER
 INFORMED CONSENT FOR DERMAL FILLER (Injection of collagen, hyaluronic acid or other filler materials) INTRODUCTION Dermal fillers are injected just under the skin s surface in order to temporarily correct
INFORMED CONSENT FOR DERMAL FILLER (Injection of collagen, hyaluronic acid or other filler materials) INTRODUCTION Dermal fillers are injected just under the skin s surface in order to temporarily correct
3M Cavilon No Sting Barrier Film. Realize the potential of proven protection.
 3M Cavilon No Sting Barrier Film Realize the potential of proven protection. The significance of healthcareacquired skin damage. Healthcare-acquired skin damage represents negative clinical outcomes resulting
3M Cavilon No Sting Barrier Film Realize the potential of proven protection. The significance of healthcareacquired skin damage. Healthcare-acquired skin damage represents negative clinical outcomes resulting
Operating Instructions
 Operating Instructions (Household) Rechargeable Shaver Model No. ES LF71 Before operating this unit, please read these instructions completely and save them for future use. ES-LF71_AUS.indb 1 2011/02/25
Operating Instructions (Household) Rechargeable Shaver Model No. ES LF71 Before operating this unit, please read these instructions completely and save them for future use. ES-LF71_AUS.indb 1 2011/02/25
APPLICANT/BODY ART ESTABLISHMENT PERMIT STATEMENT OF CONSENT
 9. Provide the Following With Application: A. (New & Renewal Applications) Present original and provide copy of Business Certificate issued by the Everett City Clerk under provisions ofmgl c. 110 subsection
9. Provide the Following With Application: A. (New & Renewal Applications) Present original and provide copy of Business Certificate issued by the Everett City Clerk under provisions ofmgl c. 110 subsection
Instruction of B108 Portable Diamond Dermabrasion Unit
 Instruction of B108 Portable Diamond Dermabrasion Unit Preface The Micro-crystal Dermabrasion was designed by Italian Florence's Mattioli at first, until now had over 20 years history. This kind of technology
Instruction of B108 Portable Diamond Dermabrasion Unit Preface The Micro-crystal Dermabrasion was designed by Italian Florence's Mattioli at first, until now had over 20 years history. This kind of technology
Management Plan for Employee Right-to-Know (ERK)
 Management Plan for Employee Right-to-Know (ERK) Table of Contents: Annual Review Form 1.0 Purpose 2.0 Globally Harmonized System (GHS) Compliance 3.0 Identification of Workplace Hazards 4.0 Hazard Assessments
Management Plan for Employee Right-to-Know (ERK) Table of Contents: Annual Review Form 1.0 Purpose 2.0 Globally Harmonized System (GHS) Compliance 3.0 Identification of Workplace Hazards 4.0 Hazard Assessments
365 Wound Care Range. Cutting the Cost of Healthcare. 365healthcare.com
 365 Wound Care Range Cutting the Cost of Healthcare Foam Dressings Super Absorbent Dressings Film Dressings IV Dressings Island Dressings Wound Closure Strips Non-Adherent Dressings 365healthcare.com CONTENTS
365 Wound Care Range Cutting the Cost of Healthcare Foam Dressings Super Absorbent Dressings Film Dressings IV Dressings Island Dressings Wound Closure Strips Non-Adherent Dressings 365healthcare.com CONTENTS
CLIENT QUESTIONNAIRE TODAY S DATE: SPECIFIC CONCERNS REGARDING YOUR SKIN (CHECK ALL THAT APPLY) I AM INTERESTED PRIMARILY IN:
 CLIENT QUESTIONNAIRE TODAY S DATE: NAME: DATE OF BIRTH: SPECIFIC CONCERNS REGARDING YOUR SKIN (CHECK ALL THAT APPLY) Fine Lines/Wrinkles Dark Circles Puffy Eyes Blotchiness/Discoloration Uneven Skin Tone
CLIENT QUESTIONNAIRE TODAY S DATE: NAME: DATE OF BIRTH: SPECIFIC CONCERNS REGARDING YOUR SKIN (CHECK ALL THAT APPLY) Fine Lines/Wrinkles Dark Circles Puffy Eyes Blotchiness/Discoloration Uneven Skin Tone
Informed Consent Injectable Fillers
 Informed Consent Injectable Fillers INSTRUCTIONS This is an informed-consent document which has been prepared to help your plastic surgeon inform you concerning Juvederm & Juvederm Ultra Plus with Lidocaine
Informed Consent Injectable Fillers INSTRUCTIONS This is an informed-consent document which has been prepared to help your plastic surgeon inform you concerning Juvederm & Juvederm Ultra Plus with Lidocaine
Your Step-by-Step Guide
 SC (abatacept): Your Step-by-Step Guide How to prepare, use and dispose of the ORENCIA prefilled ULTRASAFE PASSIVE TM syringe in 5 main steps ORENCIA (abatacept) is a registered trademark of Bristol-Myers
SC (abatacept): Your Step-by-Step Guide How to prepare, use and dispose of the ORENCIA prefilled ULTRASAFE PASSIVE TM syringe in 5 main steps ORENCIA (abatacept) is a registered trademark of Bristol-Myers
The fight against infection starts at home.
 The fight against infection starts at home. What is a surgical site infection? There are many microorganisms (germs) that live on our skin and in the environment around us. Very few of these microorganisms
The fight against infection starts at home. What is a surgical site infection? There are many microorganisms (germs) that live on our skin and in the environment around us. Very few of these microorganisms
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Professional Hair Removal System
 pearl PRO Professional Hair Removal System Carefully read this manual before using this appliance. Ensure that you know how the appliance functions and how to operate it. Instructions for Use 1 Safety
pearl PRO Professional Hair Removal System Carefully read this manual before using this appliance. Ensure that you know how the appliance functions and how to operate it. Instructions for Use 1 Safety
PATIENT INFORMATION LEAFLET. AMETOP GEL 4% w/w Tetracaine
 PATIENT INFORMATION LEAFLET AMETOP GEL 4% w/w Tetracaine Read this entire leaflet carefully because it contains important information for you. This medicine is available without prescription. However,
PATIENT INFORMATION LEAFLET AMETOP GEL 4% w/w Tetracaine Read this entire leaflet carefully because it contains important information for you. This medicine is available without prescription. However,
PharmChek Drugs of Abuse Sweat Patch Training Manual
 PharmChek Drugs of Abuse Sweat Patch Training Manual For the Application, Removal, Specimen collection, and Transport of the PharmChek Drugs of Abuse Patch For Professional Use Only Revised November, 2016
PharmChek Drugs of Abuse Sweat Patch Training Manual For the Application, Removal, Specimen collection, and Transport of the PharmChek Drugs of Abuse Patch For Professional Use Only Revised November, 2016
Scabies Identification, Treatment and Environmental Cleaning
 Scabies Identification, Treatment and Environmental Cleaning Level III Purpose The purpose of this procedure is to treat residents infected with and sensitized to Sarcoptes scabiei and to prevent the spread
Scabies Identification, Treatment and Environmental Cleaning Level III Purpose The purpose of this procedure is to treat residents infected with and sensitized to Sarcoptes scabiei and to prevent the spread
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Operating Instructions. Model No. ES-LF70. (Household) AC/Rechargeable Shaver. English 17
 Operating Instructions (Household) AC/Rechargeable Shaver Model No. ES-LF70 2 English 17 Before operating this unit, please read these instructions completely and save them for future use. 2 3 4 1 2 3
Operating Instructions (Household) AC/Rechargeable Shaver Model No. ES-LF70 2 English 17 Before operating this unit, please read these instructions completely and save them for future use. 2 3 4 1 2 3
The OZAKI VRec Sizer (13, 15, 17 mm) JD-001-P FOR The OZAKI VRec Sizer System INSTRUCTIONS FOR USE
 The OZAKI VRec Sizer (13, 15, 17 mm) JD-001-P FOR The OZAKI VRec Sizer System INSTRUCTIONS FOR USE The OZAKI VRec Sizer (Ref: JD-001-P) Rx Only CAUTION: Federal law (USA) restricts this device to sale
The OZAKI VRec Sizer (13, 15, 17 mm) JD-001-P FOR The OZAKI VRec Sizer System INSTRUCTIONS FOR USE The OZAKI VRec Sizer (Ref: JD-001-P) Rx Only CAUTION: Federal law (USA) restricts this device to sale
Skin tears and haematoma. Janice Bianchi MSc, BSc, RGN, RMN, Pg Cert Ed
 Skin tears and haematoma Janice Bianchi MSc, BSc, RGN, RMN, Pg Cert Ed Aims Review changes in skin associated with ageing Discuss best practice in relation to: Skin tears Haematoma Compromised Barrier
Skin tears and haematoma Janice Bianchi MSc, BSc, RGN, RMN, Pg Cert Ed Aims Review changes in skin associated with ageing Discuss best practice in relation to: Skin tears Haematoma Compromised Barrier
The Safest & Most Effective
 New Innovation The Safest & Most Effective powered by The world s leading away from home skin care company Concerns About Existing Antibacterial Soaps Professional use liquid antibacterial hand washing
New Innovation The Safest & Most Effective powered by The world s leading away from home skin care company Concerns About Existing Antibacterial Soaps Professional use liquid antibacterial hand washing
INFORMED CONSENT Juvederm INJECTION
 INSTRUCTIONS This is an informed-consent document which has been prepared to help Dr. Jennifer Geoghegan inform you concerning Juvederm (Non-Animal Stabilized Hyaluronic Acid, Allergan Aesthetics) tissue
INSTRUCTIONS This is an informed-consent document which has been prepared to help Dr. Jennifer Geoghegan inform you concerning Juvederm (Non-Animal Stabilized Hyaluronic Acid, Allergan Aesthetics) tissue
 Emerging Public Health Issues: Unnecessary Exposures to Hepatitis-C (Hep-C) Through Sharing of Needles, Illegal Tattooing and Unregulated Body Art (piercings and implants) Hepatitis C (Hep-C): Hep-C is
Emerging Public Health Issues: Unnecessary Exposures to Hepatitis-C (Hep-C) Through Sharing of Needles, Illegal Tattooing and Unregulated Body Art (piercings and implants) Hepatitis C (Hep-C): Hep-C is
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
PERFECT FILLERS TECHNIQUES FOR PHYSICIANS SWISS QUALITY
 PERFECT FILLERS TECHNIQUES FOR PHYSICIANS SWISS QUALITY OUR MISSION Suisselle is a privately owned company located in a biotech hub, Yverdon-les-Bains, Switzerland. Operating to the highest standard of
PERFECT FILLERS TECHNIQUES FOR PHYSICIANS SWISS QUALITY OUR MISSION Suisselle is a privately owned company located in a biotech hub, Yverdon-les-Bains, Switzerland. Operating to the highest standard of
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Patient Information Leaflet. Dermal Filler
 Patient Information Leaflet Dermal Filler When considering treatment with dermal fillers we want you to have a safe treatment. Some risks are unavoidable and out of your control. The following information
Patient Information Leaflet Dermal Filler When considering treatment with dermal fillers we want you to have a safe treatment. Some risks are unavoidable and out of your control. The following information
Redensity Innovation in eye circle treatment
 T H E B E S T O F H Y A L U R O N I C A C I D presents Innovation in eye circle treatment As experts in aesthetic medicine and specialising in creating and manufacturing hyaluronic acid dermal fillers
T H E B E S T O F H Y A L U R O N I C A C I D presents Innovation in eye circle treatment As experts in aesthetic medicine and specialising in creating and manufacturing hyaluronic acid dermal fillers
Plan Review Application for Tattooing or Piercing
 Plan Review Application for Tattooing or Piercing If you have questions or need further assistance please contact us. Please mail, email or deliver application to: RiverStone Health - Environmental Health
Plan Review Application for Tattooing or Piercing If you have questions or need further assistance please contact us. Please mail, email or deliver application to: RiverStone Health - Environmental Health
