Bloodborne Pathogens Exposure Control Plan. December 2003
|
|
|
- Randall Dawson
- 5 years ago
- Views:
Transcription
1 Bloodborne Pathogens Exposure Control Plan December 2003
2 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2
3 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate or minimize recognized and unrecognized occupational exposure to blood or other potentially infectious materials (OPIM). 2. Identify employees occupationally exposed to blood or other OPIM while performing their regular job duties. 3. Provide employees exposed to blood and OPIM information and training. A copy of this plan is available to all employees. 4. Comply with OR-OSHA Bloodborne Pathogen Standard, (OAR) POLICY: All employees and workers will practice universal precautions when there is a potential for exposure to blood and OPIM. A. COMPLIANCE METHODS Universal Precautions, hand washing, and other engineering and work practice controls will be in place to eliminate or minimize exposure of employees. Where occupational exposure remains after instituting these controls, personal protective equipment will be used. Employees found not in compliance are subject to disciplinary action. The following methods of compliance will be observed: 1. Universal Precautions Universal Precautions, a method of infection control in which all human blood and other potentially infectious materials (OPIM) are treated as if known to be infectious regardless of the perceived status, will be observed by employees. 2. Hand Washing Readily accessible hand washing facilities with soap, warm water and paper towels are located in labs and restrooms near work areas. Antiseptic hand cleansers or towelettes will be used when hand-washing facilities are not immediately accessible. Hand hygiene will be practiced promptly and thoroughly between contacts and after contact with blood, OPIM, equipment or articles contaminated by them. B. ENGINEERING AND WORK PRACTICE CONTROLS 1. Shipments of blood or OPIM will be refused and not received if there are visible or apparent signs of leakage. Once received, only employees trained in bloodborne pathogens and appropriate PPE shall handle packages with visible or apparent signs of leakage. 2. The storage, transport and shipping of any blood or OPIM must be packaged, contained and accomplished in a manner that prevents leakage during collection, handling, processing storage, transport or shipping. 3. Refrigerators and freezers containing blood and other potentially infectious materials, and other containers used to store, transport, or ship blood or other potentially infectious H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 3
4 materials must have a bio-hazard label or be packaged in a red bag or container. Blood or specimen containers only need a label identifying their contents as long as the container does not leave the facility. Labels may be attached to each object by adhesive, string, wire, or another method to prevent loss or unintentional removal of the label. 4. Eating, drinking, smoking, applying cosmetics or lip balm and handling contact lenses are not permitted in work areas where there is a reasonable risk of occupational exposure. 5. Food and drink may not be kept in refrigerators, freezers, shelves, and cabinets or on counter tops or bench tops where blood or OPIM are present. 6. Blood exposures are to be minimized by performing all procedures in a way that minimizes splashing, spraying and spattering. 7. Mouth pipeting/suctioning of blood or OPIM is prohibited. C. PERSONAL PROTECTIVE EQUIPMENT: Personal Protective Equipment (PPE) shall be readily available to the workplace in appropriate sizes or issued to employees who work with blood or OPIM. PPE will be worn during procedures and activities that are likely to generate contact with blood or OPIM. PPE includes: Splashproof Eyewear Disposable masks Disposable gloves Disposable lab coats, gowns or aprons Disposable sharps containers Red "Biohazard" bags Broom / dustpan Additional PPE may be considered as necessary to protect workers in special situations or settings. Precautions will be followed and equipment used consistently. a. All PPE will be repaired or replaced as necessary to maintain its effectiveness. b. Gloves must be worn where it can be reasonably anticipated that the employee will have contact with blood or OPIM. Gloves shall also be worn when handling or touching contaminated items or surfaces. Disposable gloves (single use gloves) used for procedures shall not be washed or decontaminated for re-use, but shall be replaced as soon as practical, when contaminated or as soon as feasible, if they are torn, punctured or barrier function is otherwise compromised. c. Utility gloves used for housekeeping services may be decontaminated for re-use if the integrity of the glove is not compromised. They must be discarded when they are cracked, peeling, torn, punctured, or exhibit other signs of deterioration or compromised barrier function. Use of Personal Protective Equipment: Gloves: Don gloves when there is a potential for contact with blood or OPIM. Change gloves between procedures or activities involving contact with blood, OPIM, equipment or contaminated articles. Remove gloves after contact. Do not wear the same pair of gloves more once and do not wash or reuse gloves between uses. H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 4
5 Decontaminate hands after removing gloves. The integrity of the glove may have been compromised during tasks. Disposable Coat, Gown, Apron or Coverall: Wear a clean disposable coat, gown, apron or coverall to protect skin and prevent soiling of clothing during procedures that are likely to generate splashing, spraying, spattering of blood or OPIM. Remove and dispose of the coat, gown, apron or coverall prior to leaving the work area. Eye Protection, Mask, Face Shield: Wear splashproof eyewear and a mask or a mask with face shield to protect mucous membranes of the eyes, nose, and mouth during procedures and activities that are likely to generate contact with blood or OPIM. Dispose of mask or mask with faceshield and disinfect splashproof eyewear prior to leaving the work area. D. HOUSEKEEPING All work areas must be maintained in clean and sanitary condition in accordance with a written schedule for cleaning and method of decontamination with an EPA approved disinfectant agent, of each area where there is blood or OPIM present. The schedule and method will be posted in a conspicuous place and include: a. All equipment and environmental and working surfaces shall be cleaned and decontaminated after contact with blood or OPIM. o Immediately or as soon as feasible after incidental spill of blood or OPIM. o At the end of each work shift if the surface may have become contaminated since last cleaning. b. All equipment, pails, cans, similar receptacles and reusable supplies intended for reuse which have a reasonable likelihood of becoming contaminated with blood or OPIM shall be inspected and decontaminated on a regularly scheduled basis, and cleaned and decontaminated immediately or as soon as feasible upon visible contamination. These items will be cleaned with disinfectant per manufacturers instructions or a bleach solution of 1 part bleach to 10 parts water. Decontaminated items may be double bagged and discarded in normal waste disposal. c. Broken glass, which may be contaminated, shall not be picked up directly with the hands. The employee must use mechanical means such as brush and dustpan, tongs or forceps. d. Sharps such as broken glass vials, bore tubes, centrifuge tubes, beakers, stir bars etc. which have been contaminated with human blood or OPIM will be placed in a properly labeled or color coded puncture resistant, leak proof sharps container. The sharps container will be sealed prior to disposal into the red biohazard bag. Transporting Blood or OPIM: Blood component containers will be labeled and transported according to OSHA standards: The storage, transport and shipping of Blood Platelets must be packaged, contained and accomplished in a manner that prevents leakage during collection, handling, processing storage, transport or shipping. Gloves must be worn where it can be reasonably anticipated that the employee will have contact with blood or other potentially infectious materials (OPIM). Person(s) responsible for transporting specimens will refuse receipt of a blood component container if it is leaking or shows signs of previous leakage. H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 5
6 Infectious Waste Disposal: Contain and secure infectious articles in a red impervious bag, e.g. any item that will splash or seep body fluid when handled or compressed. Double bagging is only necessary when the outside of the bag is also contaminated with body fluids. Call Bio Med at to have the bag picked up and disposed of. Bio Med will leave a new bag in its place. Sharp Items Disposal: Place sharp items such as broken glass, bore tubing, syringes or other sharp items in appropriate puncture-resistant sharps containers that are: o Not filled above the maximum fill line o Located as close as practical to the area in which the item was used. Do not remove any items from sharps container Place sharps container into red biohazard bag. Call Bio Med at to have the bag picked up and disposed of. Bio Med will leave a new bag in its place. E. EXPOSURE: In the event of a spill, anyone not involved in the cleanup will exit the spill area to avoid contact with blood or OPIM. Mark the affected area with a wet floor sign and cover the spill with paper towels. If an exposure occurs (pathogens enter body through eyes, wound or sharp object penetration): wash vigorously and thoroughly with soap and water for at least 10 seconds. If the eyes are involved, rinse eyes for at least 5 minutes. Notify the Supervisor or Safety Committee Member to report the location, time, and approximate size of the spill. Seek immediate medical attention through Occupational Medicine or Albany General Hospital. The attending physician will determine the level of exposure and required treatment. Post exposure HIV vaccine is effective if administered within 2 hours of exposure. Post exposure Hepatitis vaccine is effective if administered within 72 hours of exposure. H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 6
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
BLOODBORNE PATHOGENS
 MARICOPA COUNTY SHERIFF S OFFICE POLICY AND PROCEDURES Subject Related Information CRITICAL POLICY PURPOSE BLOODBORNE PATHOGENS Supersedes CP-6 (08-14-15) Policy Number CP-6 Effective Date 11-22-16 The
MARICOPA COUNTY SHERIFF S OFFICE POLICY AND PROCEDURES Subject Related Information CRITICAL POLICY PURPOSE BLOODBORNE PATHOGENS Supersedes CP-6 (08-14-15) Policy Number CP-6 Effective Date 11-22-16 The
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
Bloodborne Pathogens Safety in Your Workplace
 Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Technical Information. Clorox Healthcare Bleach Germicidal Cleaner OVERVIEW. Clorox Healthcare Bleach Germicidal
 OVERVIEW Bleach Germicidal Cleaner is engineered as a ready-to-use sporicidal cleaner-disinfectant system for healthcare facilities. This EPA-registered disinfectant contains sodium hypochlorite and other
OVERVIEW Bleach Germicidal Cleaner is engineered as a ready-to-use sporicidal cleaner-disinfectant system for healthcare facilities. This EPA-registered disinfectant contains sodium hypochlorite and other
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
SKIDMORE COLLEGE. Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS. Introduction -- Pg. 2
 SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
The methods of implementation of these elements of the standard are discussed in the subsequent pages of this ECP.
 Calvin College Bloodborne Pathogens Exposure Control Plan POLICY Calvin College is committed to providing a safe and healthful work environment for our entire staff. In pursuit of this endeavor, the following
Calvin College Bloodborne Pathogens Exposure Control Plan POLICY Calvin College is committed to providing a safe and healthful work environment for our entire staff. In pursuit of this endeavor, the following
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Spring 2005 Pollution Prevention Workshop For Healthcare
 Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN
 ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
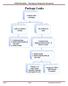 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION
 TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION 1. GENERAL PRACTITIONER INFORMATION New Registration Annual Registration Updated Registration FULL LEGAL NAME (Give
TATTOOING, BODY PIERCING, PERMANENT COSMETICS & BRANDING APPLICATION FOR REGISTRATION 1. GENERAL PRACTITIONER INFORMATION New Registration Annual Registration Updated Registration FULL LEGAL NAME (Give
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
(c) BODY ART ESTABLISHMENT means any location, whether temporary or permanent, where the practices of body art are performed.
 DEPARTMENT OF PUBLIC HEALTH AND ENVIRONMENT Division of Environmental Health and Sustainability BODY ART ESTABLISHMENTS 6 CCR 1010-22 [Editor s Notes follow the text of the rules at the end of this CCR
DEPARTMENT OF PUBLIC HEALTH AND ENVIRONMENT Division of Environmental Health and Sustainability BODY ART ESTABLISHMENTS 6 CCR 1010-22 [Editor s Notes follow the text of the rules at the end of this CCR
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
rooo.lb IOWA COUNTY ORDINANCE NO TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS:
 .. rooo.lb IOWA COUNTY ORDINANCE NO. 4-196 TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS: SECTION I: The following ordinance of Iowa County, Wisconsin is hereby created
.. rooo.lb IOWA COUNTY ORDINANCE NO. 4-196 TATTOO ARTIST REGULATIONS THE IOWA COUNTY BOARD OF SUPERVISORS DO ORDAIN AS FOLLOWS: SECTION I: The following ordinance of Iowa County, Wisconsin is hereby created
Hand Hygiene ORGANIZATIONAL: Affects two or more departments.
 Hand Hygiene ORGANIZATIONAL: Affects two or more departments. Folder Infection Prevention Sub-Folder Original 1/1/1987 Scope All Effective Date Approved (Approver/Date) Last Reviewed/ Revised Date IPC:
Hand Hygiene ORGANIZATIONAL: Affects two or more departments. Folder Infection Prevention Sub-Folder Original 1/1/1987 Scope All Effective Date Approved (Approver/Date) Last Reviewed/ Revised Date IPC:
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
Hand Hygiene. Policy Title: Hand Hygiene Policy Number: 05. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Hand Hygiene 1. POLICY STATEMENT: 1.1. Applies to what is the best practice in hand hygiene. 2. PURPOSE: 2.1. To prevent/minimize the risk of infection in dental settings. 2.2. To promote awareness for
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
APPROVAL REVIEW PROCEDURES
 Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Safe Handling and Disposal of Sharps. Reference Guide
 Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
BODY ART GUIDELINES. Purpose. Definitions. Body Art Technician Requirements
 BODY ART GUIDELINES Purpose This guideline provides general explanations of procedures for the maintenance and operation of body art facilities and permitting requirements for body art technicians. Please
BODY ART GUIDELINES Purpose This guideline provides general explanations of procedures for the maintenance and operation of body art facilities and permitting requirements for body art technicians. Please
Permanent Body Art Facility Plan Review Application
 Permanent Body Art Facility Plan Review Application Livingston County Health Department 2300 East Grand River Suite 102, Howell, MI 48843 Ph:517-546-9858 Fx:517-546-9853 www.lchd.org Authority - Michigan
Permanent Body Art Facility Plan Review Application Livingston County Health Department 2300 East Grand River Suite 102, Howell, MI 48843 Ph:517-546-9858 Fx:517-546-9853 www.lchd.org Authority - Michigan
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions
 Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
LAPORTE COUNTY TATTOO & BODY PIERCING ORDINANCE
 LAPORTE COUNTY TATTOO & BODY PIERCING ORDINANCE 2011-07 1 Ordinance No. 2011-07 OF THE BOARD OF COMMISSIONERS OF LAPORTE COUNTY, INDIANA Whereas, the Indiana State Department of Health has promulgated
LAPORTE COUNTY TATTOO & BODY PIERCING ORDINANCE 2011-07 1 Ordinance No. 2011-07 OF THE BOARD OF COMMISSIONERS OF LAPORTE COUNTY, INDIANA Whereas, the Indiana State Department of Health has promulgated
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ]
![ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ] ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST. Trained Observer: Unit: Date: [ ] the floor. 2 Engage Trained Observer and Assistant [ ]](/thumbs/78/77108755.jpg) Page 1 of 5 ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST Trained Observer: Unit: Date: Healthcare Worker: Assistant: Instructions: Check box after completion of each step. Donning of HEALTHCARE
Page 1 of 5 ENHANCED CONTACT AND DROPLET PRECAUTIONS PPE CHECKLIST Trained Observer: Unit: Date: Healthcare Worker: Assistant: Instructions: Check box after completion of each step. Donning of HEALTHCARE
WHISTON WORRYGOOSE JUNIOR AND INFANT SCHOOL
 WHISTON WORRYGOOSE JUNIOR AND INFANT SCHOOL Part of White Woods Academy Trust BODILY FLUID HYGIENE POLICY Approved by Governors: September 2017 Review Date: September 2019 Statement of intent At Whiston
WHISTON WORRYGOOSE JUNIOR AND INFANT SCHOOL Part of White Woods Academy Trust BODILY FLUID HYGIENE POLICY Approved by Governors: September 2017 Review Date: September 2019 Statement of intent At Whiston
SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS
 SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS Purpose To ensure the safe disposal of potentially contaminated sharps, syringes, clothing and any other waste products. Scope
SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS Purpose To ensure the safe disposal of potentially contaminated sharps, syringes, clothing and any other waste products. Scope
Welcome to the Hazard Communication Course
 Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
BODY ART FACILITY CONSTRUCTION PLAN CHECK
 BODY ART FACILITY CONSTRUCTION PLAN CHECK Type of Facility: (mark one) Permanent Temporary/Special Event Are you a: (mark one) New Facility Existing with new ownership Existing Facility Existing remodel
BODY ART FACILITY CONSTRUCTION PLAN CHECK Type of Facility: (mark one) Permanent Temporary/Special Event Are you a: (mark one) New Facility Existing with new ownership Existing Facility Existing remodel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
What is infection control?
 Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Competency. Method of Instruction Codes. Yes / No / RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN. Yes No RDN.
 JOHNS HOPKINS MEDICAL INSTITUTIONS Competency Validation Tool BIOLOGICAL PPE ENHANCED CONTACT AND DROPLET PRECAUTIONS (POWERED AIR PURIFYING RESPIRATOR (PAPR) OPTION) Employee Name: Role: Unit: Date: DESIRED
JOHNS HOPKINS MEDICAL INSTITUTIONS Competency Validation Tool BIOLOGICAL PPE ENHANCED CONTACT AND DROPLET PRECAUTIONS (POWERED AIR PURIFYING RESPIRATOR (PAPR) OPTION) Employee Name: Role: Unit: Date: DESIRED
Hygienic requirements for tattoo and piercing studios
 Hygienic requirements for tattoo and piercing studios Activities injuring the skin or mucus membrane are linked to an increased infection risk for diseases transferred by blood and serum. To avoid transferable
Hygienic requirements for tattoo and piercing studios Activities injuring the skin or mucus membrane are linked to an increased infection risk for diseases transferred by blood and serum. To avoid transferable
Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5
 Environmental Health and Safety Manual Policy Number: EH&S 4-4 Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5 PURPOSE: To establish safe handling practices and use of
Environmental Health and Safety Manual Policy Number: EH&S 4-4 Title: Formaldehyde Safety Effective Date: 10/94 Revision: 2/97 Number of Pages: 5 PURPOSE: To establish safe handling practices and use of
