Roosevelt Biosafety Training. Created 10/2015
|
|
|
- Rebecca Wilkinson
- 6 years ago
- Views:
Transcription
1 Roosevelt Biosafety Training Created 10/2015
2 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them Learn ways to reduce risks and hazards by understanding the use of aseptic technique and proper use of biological safety cabinets Learn proper procedures for cleaning biohazardous spills Learn proper disposal procedures for biohazardous waste in biological laboratories
3 Identifying Risks Risk assessment: What are the biological and physical hazards of the organism/agent? What procedures may spread the organism/agent? What is the best method for inactivating and containing the organism/agent? What is the pathogenicity of the organism/agent? What are the potential deficiencies in practices of lab workers?
4 Identifying Risks Risk assessment: Identify agent hazards CDC and WHO have guidelines Biosafety level Identify laboratory procedure hazards Aerosols Volume and concentration Use of sharps or animals Complexity of experiment Determine appropriate biosafety level and additional precautions Evaluate staff and equipment for safe practices Review risk assessment with biosafety professionals
5 Identifying Risks Risk Groups Organisms are categorized by: Potential effect of the agent on a healthy human adult* Ease and route of transmission Infective dose Stability in environment Knowing the risk group of the organism can determine the procedures necessary for reducing risk May differ between strains of the same organism, depending on pathogenicity of the different strains NIH and WHO definitions differ slightly Do not equal biosafety levels (though they are correlated)
6 Risk Groups Risk Group 1 Agents not associated with disease in humans or animals Risk Group 2 Agents associated with human or animal disease, but are unlikely to cause serious hazard to lab workers If exposure happens, preventive or therapeutic interventions are usually available Risk Group 3 Agents associated with serious or lethal human or animal disease Treatments may exist, and the agent isn t easily spread Use is discouraged at Roosevelt University Risk Group 4 Agents associated with serious or lethal human or animal disease that can be easily passed between individuals Treatments are not usually available Use is prohibited at Roosevelt University
7 Identifying Risks Routes of laboratory transmission Inoculation from contaminated sharps Spills and splashes onto skin and mucous membranes Ingestion (mouth pipetting) Animal bites and scratches Inhalation of infectious aerosols Considered to be a serious hazard and likely culprit of most laboratory exposures
8 Reducing Risks: Aerosols Procedures that produce aerosols: Pipetting Blending Centrifugation Sonicators and vortex mixers Vigilant workers can reduce the amount of aerosols by being cautious with above procedures Use biological safety cabinet when these procedures must be performed
9 Reducing Risks: PPE Minimum Personal Protective Equipment: Safety glasses Gloves Lab coat Other equipment that may be necessary based on risk assessment: Mask or face shield Respirator Gown
10 Reducing Risk: Equipment Biological Safety Cabinets (BSC) Produce a sterile field to protect both the worker and the culture Centrifuge safety cups Sealed rotors Maintenance of equipment is essential
11 Reducing Risk: Facility Engineering controls to prevent release of hazards Directional airflow in the lab Substitution of lower risk agent for higher risk agent if possible Limited access to building or labs Training
12 Biosafety Level 1 Well characterized strains of agents not known to cause disease in healthy adults Basic containment with sink for hand washing Laboratory doors kept closed during experiments Decontaminate work surfaces daily and after spills All waste decontaminated before disposal Mouth pipetting prohibited Eating, drinking, smoking, and applying cosmetics are not permitted in lab Wash hands after handling materials and when leaving the lab PPE use recommended (required at Roosevelt) Spills reported to lab manager to ensure proper documentation and clean-up
13 Biosafety Level 2 All as in BSL-1 Moderate-risk agents Can be used on the open bench if risk of aerosols and splashes is low, higher risk organisms must be manipulated in BSC PPE required, cannot be worn in non-laboratory areas Access to sink for hand washing and decontamination Access to laboratory is limited or restricted Persons with increased risk of acquiring infection or for whom infection is unusually hazardous should not be allowed into labs Only individuals that meet entry requirements (e.g. immunizations) may enter Workers advised of potential hazards and given proper training in handling agents Biohazard signs clearly posted on doors and equipment Sharps use minimized
14 Biosafety Level 3 All as in BSL-1 and -2 Agents with a potential for respiratory transmission with serious or lethal infections Work is discouraged at Roosevelt Lab personnel must have specific training in handling agents and are supervised while conducting experiments All work done in BSC or other enclosed equipment Controlled access to lab and special ventilation to prevent accidental release Lab must have specific design and containment equipment Air lock, shower, or changing room required between unrestricted areas and lab Surfaces of walls, floors, and ceilings must be water resistant Windows are closed and sealed Exhaust system provided to prevent release of agents PPE required and may include respirator, double gloves, gowns, etc. Lab coats are not suitable Workers must comply with entry and exit procedures Vacuum lines protected with HEPA filters and liquid traps
15 Biosafety Levels 4 All as in BSL-1, -2, and -3 Work is prohibited at Roosevelt Agents with high risk of lethal disease, easily transmitted (aerosol), with little or no treatment options Class III BSC or full-body, air-supplied positive pressure suit required for working Controlled access to labs, specialized ventilation, and waste management systems
16 Biosafety Cabinets Use when procedures are likely to produce aerosols or when high concentrations or large volumes of agent are being used Different models provide protection in different ways; must be chosen according to needs of the lab and agents used Lab workers must be trained in proper use of BSC
17 Biosafety Cabinets For proper use of BSC, watch following video: Lab workers may require in-person training depending on procedures, experience, and agents being used Instructors MUST train their students (students cannot train other students) In-person training will be provided by instructor or laboratory manager
18 Aseptic Technique Method of laboratory work that prevents contamination by (unwanted) microorganisms Provides barrier between sterile cell cultures and microorganisms in the environment Varies depending on whether working on the bench or in a BSC
19 Aseptic Technique For proper bench-top aseptic technique, watch the following video: Lab workers may require in-person training depending on procedures, experience, and agents being used Instructors MUST train their students (students cannot train other students) In-person training will be provided by instructor or laboratory manager
20 Biohazardous Spills Basic biological spill kit should contain: Disinfectant (e.g. bleach 1:10 dilution, diluted quaternary solution, or other suitable disinfectant) Absorbent material (paper towels, spill pillows, etc.) Waste container (biohazard bags and sharps containers) PPE Mechanical tools (forceps, dustpan and broom) Procedure can depend on agent spilled and where
21 Biohazardous Spills BSL-1 spills: Notify others Wear PPE Surround spill with disinfectant Clean up with paper towels (if large, use spill pillows) Re-apply disinfectant to the surface and let sit for 10 minutes. Clean again. Put contaminated waste in biohazard bags for autoclaving Wash hands Notify lab manager to assure proper cleanup
22 Biohazardous Spills BSL-2 spills: Evacuate the room and close doors; notify lab manager Remove any contaminated clothing and decontaminate body surfaces Allow at least 30 minutes for potential aerosols to be reduced before reentering Don protective clothing and respiratory protective equipment Decontaminate spill with appropriate disinfectant and allow 10 minutes of contact time Clean spill with paper towels or spill pillows and dispose in biohazard bag Pick up sharps with forceps or tweezers, never with hands, and dispose of in autoclavable sharps container Reapply disinfectant and clean after 10 minutes. Wash hands and/or shower after cleaning spill
23 Biohazardous Spills Biosafety Cabinet Spill Keep cabinet running during the cleanup Remove any contaminated PPE and replace with clean Apply appropriate disinfectant to the spill (bleach can be used but should be used with caution; it will corrode the stainless steel) Wipe up spill and dispose of paper towels in biohazard bag Reapply disinfectant and clean again If bleach is used, clean the surface of the cabinet with water to remove traces of bleach Items that must be removed should also be decontaminated before unloading from cabinet Run UV/germicidal lamp for at least 15 minutes for final decontamination (formaldehyde gas can also be used)
24 Waste Disposal Sharps Items capable of puncturing, cutting, or abrading the skin (e.g. broken glass or plastic ware, scalpels, razor blades, needles, etc.) Never place sharps in regular trash Dispose of in puncture proof containers Clean broken glass can go into broken glass containers Any sharp contaminated with blood or other biohazard must be decontaminated (autoclave or bleach) and disposed of in an appropriate container Leak proof, rigid, puncture-resistant Tightly sealed Labeled with biohazard symbol
25 Waste Disposal Biohazardous waste Waste containing infectious or potentially infectious substances (e.g. blood, bacterial cultures, liquid waste from cell culture, etc.) All waste must be disposed of in bags marked with biohazard symbol; bags can go into labeled, leak-proof containers to await autoclaving Autoclaved before disposal in regular trash Autoclave should be checked regularly for proper functioning (reaches temperature and pressure, etc.)
26 Obtaining Biohazardous Materials Lab Manager approval required for new organisms Check risk group and recommended biosafety level Determine if necessary or if a lower-risk organism can be used instead Submit for lab manager approval Will check requirements to determine if Roosevelt has appropriate facilities Currently no facilities for BSL-3 Fill out required paperwork in order to obtain organism Training with new organism must be conducted by lab manager or instructor
27 Resources and Sources Center for Disease Control s Biosafety site - NIH Office of Science Policy for Biosafety - Roosevelt s CHP - /Safety/ChemicalHygienePlan.ashx
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
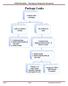 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard
 ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
Section 4 Procedures for Biohazard Control
 Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
Page 4-1 Section 4 Procedures for Biohazard Control Contents SECTION 4 PROCEDURES FOR BIOHAZARD CONTROL... 4-1 A. FACILITY REQUIREMENTS... 4-3 1. BSL-1 Laboratory Facilities... 4-3 2. BSL-2 Laboratory
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could happen? What is the worst thing that could happen? What can
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS
 PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
APPROVAL REVIEW PROCEDURES
 Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
GUIDELINES FOR THE IMPLEMENTATION AND ENFORCEMENT OF BOSTON PUBLIC HEALTH COMMISSION S BODY ART REGULATIONS
 GUIDELINES FOR THE IMPLEMENTATION AND ENFORCEMENT OF BOSTON PUBLIC HEALTH COMMISSION S BODY ART REGULATIONS APPROVED: Introduction Monica Valdes Lupi Executive Director Revised: September 19, 2017 The
GUIDELINES FOR THE IMPLEMENTATION AND ENFORCEMENT OF BOSTON PUBLIC HEALTH COMMISSION S BODY ART REGULATIONS APPROVED: Introduction Monica Valdes Lupi Executive Director Revised: September 19, 2017 The
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
Bloodborne Pathogens Safety in Your Workplace
 Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
BODY ART FACILITY CONSTRUCTION PLAN CHECK
 BODY ART FACILITY CONSTRUCTION PLAN CHECK Type of Facility: (mark one) Permanent Temporary/Special Event Are you a: (mark one) New Facility Existing with new ownership Existing Facility Existing remodel
BODY ART FACILITY CONSTRUCTION PLAN CHECK Type of Facility: (mark one) Permanent Temporary/Special Event Are you a: (mark one) New Facility Existing with new ownership Existing Facility Existing remodel
Welcome to the Hazard Communication Course
 Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET EPOXICONAZOLE TC 1. Product & Manufacturer information Product Name: EPOXICONAZOLE TECH Manufacturer: Shanghai Tenglong Agrochem Co., Ltd. Phone Numbers: Tel: 86-21 5506 3225
MATERIAL SAFETY DATA SHEET EPOXICONAZOLE TC 1. Product & Manufacturer information Product Name: EPOXICONAZOLE TECH Manufacturer: Shanghai Tenglong Agrochem Co., Ltd. Phone Numbers: Tel: 86-21 5506 3225
Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan
 Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan (last revised Aug 30, 2011) The Maurice Kanbar Center for Biomedical Engineering Room 704 NAB David Wootton, Ph.D. Director
Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan (last revised Aug 30, 2011) The Maurice Kanbar Center for Biomedical Engineering Room 704 NAB David Wootton, Ph.D. Director
Standard Operating Procedure: Hydrofluoric Acid. Copyright Drexel University Health and Safety
 Standard Operating Procedure: Hydrofluoric Acid Copyright Drexel University Health and Safety Hydrofluoric Acid (HF) Purpose of Training The Purpose of this training session is to develop safe working,
Standard Operating Procedure: Hydrofluoric Acid Copyright Drexel University Health and Safety Hydrofluoric Acid (HF) Purpose of Training The Purpose of this training session is to develop safe working,
KERN HEALTH SYSTEMS POLICIES AND PROCEDURES 2.21-P
 Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
Workplace Hazardous Materials Information System (WHMIS) Self Learning Package
 Saskatchewan Health Authority OCCUPATIONAL HEALTH & SAFETY Workplace Hazardous Materials Information System (WHMIS) 1988 Self Learning Package What is WHMIS? The Workplace Hazardous Materials Information
Saskatchewan Health Authority OCCUPATIONAL HEALTH & SAFETY Workplace Hazardous Materials Information System (WHMIS) 1988 Self Learning Package What is WHMIS? The Workplace Hazardous Materials Information
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES
 BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
Safe Handling and Disposal of Sharps
 SBC Children, Families And Community Health Service Statement of Intent Safe Handling and Disposal of Sharps To provide clear guidelines for the safe handling and disposal of all sharps in order that the
SBC Children, Families And Community Health Service Statement of Intent Safe Handling and Disposal of Sharps To provide clear guidelines for the safe handling and disposal of all sharps in order that the
Acid Or Alkali? Testing With Cabbage
 Acid Or Alkali? Testing With Cabbage Topic Using vegetables as an acid/base indicator Introduction Forensic scientists need to discover if someone has tampered with liquids (e.g., cosmetics, cleaning products,
Acid Or Alkali? Testing With Cabbage Topic Using vegetables as an acid/base indicator Introduction Forensic scientists need to discover if someone has tampered with liquids (e.g., cosmetics, cleaning products,
2006 COURSE TITLE: HAZARDOUS MATERIALS SAFETY
 COURSE INTRODUCTION Hazardous substances are routinely used at work. However, that does not mean they are without risk. Some substances harm you right away. Others may cause health problems that show up
COURSE INTRODUCTION Hazardous substances are routinely used at work. However, that does not mean they are without risk. Some substances harm you right away. Others may cause health problems that show up
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
Hazard Communication Program
 ILIULIUK FAMILY AND HEALTH SERVICES Hazard Communication Program Reviewed April 2015 TABLE OF CONTENTS SECTION ONE: Staff and General Information Overview 1 I. Introduction II. Purposes III. Responsibility
ILIULIUK FAMILY AND HEALTH SERVICES Hazard Communication Program Reviewed April 2015 TABLE OF CONTENTS SECTION ONE: Staff and General Information Overview 1 I. Introduction II. Purposes III. Responsibility
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
