Appendix C. Infectious Waste Guidelines
|
|
|
- Jeffrey Holmes
- 6 years ago
- Views:
Transcription
1 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section , is registered with the Ohio Environmental Protection Agency (OEPA) as a large-quantity generator of infectious waste. Faculty and staff who generate infectious waste must comply with OEPA regulations. For generators of infectious waste (faculty, staff, students, etc.) the following pages contain information dealing with these regulations. Individual PIs/Supervisors are responsible for assuring compliance with infectious regulations including: 1. identification and segregation; 2. proper packaging; 3. proper treatment; 4. personnel training; 5. spill and containment plans; 6. spill response; 7. spill reporting; and 8. contingency plans. It is the PI s/supervisor s responsibility to notify the Institutional Biosafety Committee, or OEHS of their activities and to comply with OEPA regulations. Assistance is available from OEHS to help develop and implement procedures consistent with the regulations. Individuals who wish to treat their own infectious waste must register with OEHS at , obtain a treatment facility permit from OEPA and undergo quarterly laboratory audits by OEHS and a representative of OEPA. Records must be kept of all waste treatment and disposal. This includes treating liquid waste with bleach and disposing in the sanitary sewer. C.1 March 2013
2 C.2 Definitions of Infectious Waste 1. Cultures and stocks of infectious agents (human pathogens) and associated biologicals, including without limitation, specimen cultures, cultures and stocks of infectious agents, wastes from production of biologicals and discarded live and attenuated vaccines; 2. Laboratory wastes that were, or are likely to have been, in contact with infectious agents that may present a substantial threat to public health if improperly managed; 3. Pathological wastes, including, without limitation, human and animal tissues, organs, and body parts, and body fluids and excreta that are contaminated with or are likely to be contaminated with infectious agents, removed or obtained during surgery or autopsy or for diagnostic evaluation provided that, with regard to pathological waste from animals, the animals have or are likely to have been exposed to a zoonotic or infectious agent; 4. Waste materials from the rooms of humans, or the enclosures of animals, that have been isolated because of diagnosed communicable diseases that are likely to transmit infectious agents. Also included are waste materials from the rooms of patients who have been placed on blood and body fluid precautions under the Universal Precaution System established by the Centers for Disease Control and Prevention in the Public Health Service of the United States Department of Health and Human Services, if specific wastes generated under the Universal Precaution System have been identified as infectious wastes by rules referred to in C.2.8 below; C.2 March 2013
3 5. Human and animal blood specimens and blood products that are being disposed of provided that with regard to blood specimens and blood products from animals, the animals were or were likely to have been exposed to a zoonotic or infectious agent. Blood products do not include patient care waste such as bandages or disposable gowns that are lightly soiled with blood or bodily fluids unless such wastes are soiled to the extent that the generator of the wastes determines that they should be managed as infectious wastes; 6. Contaminated carcasses, body parts, and bedding of animals that were intentionally exposed to infectious agents from zoonotic or human diseases during research, production of biologicals, or testing of pharmaceuticals, and carcasses and bedding of animals otherwise infected by zoonotic or infectious agents that may present a substantial threat to public health if improperly handled; 7. Sharp wastes used in the treatment, diagnosis, or inoculation of human beings or animals or that have, or are likely to have, come in contact with infectious agents in medical, research, or industrial laboratories, including, without limitation, hypodermic needles and syringes, scalpel blades, and glass articles that have been broken. Such wastes are hereinafter in this rule referred to as sharp infectious waste or sharps ; 8. Any other waste material generated in the diagnosis, treatment, or immunization of humans or animals, in research pertaining thereto, or in the production or testing of biologicals that the Public Health Council created in Section of the Revised Code, by rules adopted in accordance with Chapter 119 of the Revised Code, identifies as infectious waste after determining that the wastes represent a substantial threat to public health when improperly managed because they are contaminated, or likely to be contaminated with infectious agents. C.3 March 2013
4 C.3 Packaging, Storage and Disposal of Untreated Infectious Waste To meet Ohio Administrative Code Section for the packaging, storage and disposal of infectious waste, OSU requires the following: C.3.1 Material 1. Red bags or biohazard bags, biohazard shipping boxes, and sharps containers; 2. All material in C except sharps containers are available at no charge from EHS, excluding the Medical Center operations. Contact Environmental Services in the Hospital for additional information. Sharps containers are available through the Medical Stores. For university locations, delivery of waste supplies can be requested by logging into the EHS Online secure web application. C.3.2 Packaging 1. Assemble the infectious waste box provided by Environmental Health and Safety (OEHS) and ensure that all markings are oriented correctly with the Up Arrows pointed upward. 2. Tape all seams with sturdy packaging tape. NOTE: Masking tape is not acceptable. 3. Line the infectious waste box with the EHS provided red plastic infectious waste bag prior to placement of infectious waste materials into the container. 4. Place only infectious material or infectious contaminated materials in the infectious waste bags used to line infectious waste boxes. C.4 March 2013
5 5. Store liquid infectious waste in Department of Transportation (DOT) approved plastic containers or carboys prior to packaging for pickup. NOTE: Total liquid volume is not to exceed four (4) gallons. 6. Place liquid containing infectious waste containers in the bottom of lined infectious waste boxes to facilitate pickup and storage. NOTE: Total liquid volume is not to exceed four (4) gallons. 7. Limit the total weight in the infectious waste boxes to 30 pounds. 8. Seal the bag prior to sealing the box. 9. Seal the box securely with packaging tape. 10. Include the building name, room number and the name of the principal investigator or lab supervisor, waste request number on the top of the box. 11. Arrange for pickup of packaged infectious waste or to request storage containers or packaging materials via the EHS website ( C.3.3 Storage 1. Lock outside storage areas containing infectious waste containers to prevent unauthorized access. 2. Designate infectious waste storage areas. Those storage areas that are not locked, shall be visibly labeled with a sign stating Warning: Infectious Waste and/or displaying the international biohazard symbol on all points of access. C.5 March 2013
6 C.3.4 Disposal 1. Request pick-up of packaged infectious waste via the EHS website ( 2. Generators of infectious waste may discharge untreated liquid or semi-liquid infectious wastes consisting of blood, blood products, body fluids, and excreta into the sanitary sewer system as defined in Section of the Revised Code, unless the discharge is inconsistent with the terms and conditions of any permit for the system involved under Chapter 6111 of the Revised Code (OAC C). C.3.5 Spills 1. All individuals who use biohazard substances must record in a log all spills or accidents involving infectious waste. For spills in quantities greater than one gallon or which involve exposure of laboratory personnel, OEHS must be notified; 2. All individuals who use biohazard substances must develop and implement a spill-containment and clean-up procedure. The procedure must be readily available to persons likely to handle infectious waste; 3. Sections C.7 and C.8 are provided to meet these requirements. Modifications of procedures must be forwarded to OEHS for review and comments. C.4 Treatment by Incineration Those who wish to treat infectious waste onsite by incineration must comply with OAC In the past, infectious waste (primarily animal carcasses and bedding) had been incinerated at Wiseman Hall, Biological Sciences or Goss Laboratory. These incinerators are not permitted by the Ohio Environmental Protection C.6 March 2013
7 Agency to burn infectious waste. Administrative units responsible for these incinerators have been notified that all incineration of infectious waste must cease immediately. Infectious waste currently treated at one of these locations should be packaged according to instructions provided above in C.3. Contact OEHS at for further assistance. C.5 Treatment by Steam Sterilization 1. Those who wish to treat infectious waste onsite using steam sterilization must also comply with OAC and be permitted by OEPA. Contact Environmental Health and Safety for more information. C.6 Chemical Treatment Chemical treatment of infectious waste also requires complying with OAC and be permitted by OEPA. Contact Environmental Health and Safety for information. The Ohio Environmental Protection Agency has only approved chemical treatment of infectious waste categorized as cultures. Therefore, chemical treatment of any other category of infectious waste must be approved by the Director OEPA or an alternate approved-treatment method used. C.7 March 2013
8 C.7 Spill Containment and Clean-up Procedures According to OAC , spill containment and a clean-up kit shall be available in those areas designated in the Spill Containment and Cleanup Procedures. The location of the kits shall provide for rapid and efficient clean up of spills anywhere within these areas. C.7.1 Spill Kit Materials The kit shall include but is not limited to: 1. Absorbent; 2. One gallon approved chemical disinfectant (bleach); 3. Red bags or bags labeled with the biohazard symbol; 4. Impermeable and disposable overalls (preferably tyvek total body coveralls); 5. Gloves (heavy neoprene or latex); 6. Goggles (can be reusable); and 7. Rigid plastic container for sharps. 8. First aid kit unless emergency care is available on the premises. 9. Boundary tape and other appropriate safety equipment. C.7.2 Clean-up Procedures 1. A copy of the clean-up procedures is provided later in this section (see D.9); 2. More specific or detailed clean-up procedures can be prepared by the generator. C.8 March 2013
9 C.7.3 Spill Log 1. A copy of the spill log is also provided; 2. Spill logs must be maintained for five years; 3. All spills greater than one gallon or which involve exposure of laboratory personnel must be reported to OEHS immediately and those spills of volume greater than one cubic foot must be reported to OEHS and to the Director of OEPA within 48 hours. C.8 Contingency Plan In accordance with OAC and 35, a contingency plan for treatment facilities must be available at treatment sites. In the event that sites which treat infectious waste cannot meet the storage requirements described below or are experiencing a malfunction in treatment processes, the contingency plan shall be implemented. C.8.1 Storage 1. Store infectious waste in a manner that maintains the integrity of packing; 2. Maintain waste in a nonputrescent state, using refrigeration or freezing if necessary; 3. Lock outside storage to prevent unauthorized access; 4. Designate and label storage areas by posting biohazard warning signs; 5. Store infectious waste in a manner that affords protection from animals; 6. No infectious waste may be stored more than 14 days; C.9 March 2013
10 7. No more than seven times the treatment facility's total maximum daily throughput capacity shall be stored for treatment. 8. Contain and clean up any spill of infectious waste within a storage area using approved methods. C.10 March 2013
11 Emergency Coordinator: Telephone: Institutional Laboratory Biosafety Manual CONTINGENCY PLAN Alternate Coordinator: Michael St. Clair, EHS Telephone: If you cannot comply with the storage requirements set forth, the following contingency plan shall be implemented: a. Notify your Emergency Coordinator; b. Call OEHS and request red bags, biohazard boxes, and sharps containers as needed for packing infectious waste at your treatment location; c. Following packaging of infectious waste, OEHS will arrange for offsite incineration. 2. Listing of emergency telephone numbers in addition to the Emergency Coordinator. a. OSU Police Dispatcher: 911 from campus phone b. OEHS Chemical/Infectious Waste Management: (614) c. OEHS Main Office: (614) d. OEPA Central District Office: (614) e. Emergency Number: 911 f. Columbus Health Department: (614) CONTACT: Michael St. Clair, 1314 Kinnear Rd; Tel: (614) C.11 March 2013
12 D.9 INFECTIOUS WASTE SPILL CONTAINMENT AND CLEAN-UP PROCEDURE In accordance with OAC , the following containment and clean-up procedures are to be implemented in the event of an infectious waste spill. INFECTIOUS WASTE SPILL CONTAINMENT AND CLEAN-UP PROCEDURE Infectious waste spills must be contained and cleaned up immediately. I. A spill kit containing absorbent material, bleach or another USEPA registered tuberculocidal disinfectant, biohazard bags, gloves, eye protection, and a biohazard sharps container must be accessible in the laboratory. II. To use bleach as a disinfectant, a 1:10 dilution (minimum 10% sodium hypochlorite solution) of household bleach should be prepared immediately prior to use, with a minimum of 30 minutes contact time with the waste. If another USEPA registered tuberculocidal disinfectant is used, the manufacturer s recommendations for concentration and contact time should be followed. 1. Limit access to area to authorized personnel. 2. Open the spill kit. 3. Put on appropriate PPE (i.e. gloves, eye protection, coveralls). 4. Contain liquid spills by covering with absorbent pads. Place contaminated absorbent pads and other contaminated solids into a biohazard bag. Seal the bag by tying in a knot and place C.12 March 2013
13 into a second biohazard bag. Sharps (i.e. needles, blades or broken glassware) associated with the spill should be placed in a biohazard sharps container. 5. Clean the spill and cover contaminated surfaces with absorbent pads and soak with appropriate disinfectant (See II above). Allow the disinfectant to stand on the contaminated material for the minimum recommended contact time. 6. Place all materials used during the clean up process in a biohazard bag. Seal the bag by tying in a knot and place into a second bag. Place all biohazard bags into a biohazard burn box. 7. Disinfect all re-usable materials from the spill kit (i.e. goggles, dustpan, etc.) and put back into the kit. Replenish disposable items from the spill kit. See the OSU Institutional Biosafety Manual for additional information on Decontamination and Spills ( FOR ASSISTANCE OR QUESTIONS, CONTACT THE OFFICE OF ENVIRONMENTAL HEALTH & SAFETY AT OR THE OSU POLICE DISPATCHER AT 911 FROM A CAMPUS TELEPHONE, AFTER WORKING HOURS. C.13 March 2013
14 INFECTIOUS WASTE SPILL REPORT A spill report is required under OAC (A)(10) for any spill that is greater than or equal to one cubic foot in volume. Complete this report and return to the address listed below. Date and Time of Spill: Date of Report: Location of Spill: Employee(s) Involved in Clean-up: Waste Spilled: Estimated Quantity: Describe Clean-up Procedure: Summary of Events Causing Spill (If Known): Printed Name Signature Date Mail Completed Report To: Michael St. Clair Office of Environmental Health and Safety Room Kinnear Road, CAMPUS C.14 March 2013
Infectious Waste Contingency Plan
 Infectious Waste Contingency Plan Office of Environmental Health and Safety September 2016 Table of Contents I. Introduction..3 II.Facility Identification and Contact Information..3 III. Emergency Contacts...3-4
Infectious Waste Contingency Plan Office of Environmental Health and Safety September 2016 Table of Contents I. Introduction..3 II.Facility Identification and Contact Information..3 III. Emergency Contacts...3-4
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Spring 2005 Pollution Prevention Workshop For Healthcare
 Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Medical Waste Management Plan
 Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
KERN HEALTH SYSTEMS POLICIES AND PROCEDURES 2.21-P
 Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
MEDICAL WASTE MANAGEMENT PLAN
 MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Medical Waste Manual. California State University, Chico
 California State University, Chico The Department of Environmental Health and Safety Revised January 4, 2011 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal
California State University, Chico The Department of Environmental Health and Safety Revised January 4, 2011 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal
BODY ART ESTABLISHMENT PLANNING APPLICATION
 BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
Hazard Communication Program
 1. Purpose The University of Denver Hazard Communication Program defines the requirements and responsibilities for informing and training employees about workplace hazardous chemicals in accordance with
1. Purpose The University of Denver Hazard Communication Program defines the requirements and responsibilities for informing and training employees about workplace hazardous chemicals in accordance with
Medical Waste Manual. California State University, Chico
 California State University, Chico The Department of Environmental Health and Safety March 2017 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal of Biohazardous
California State University, Chico The Department of Environmental Health and Safety March 2017 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal of Biohazardous
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
NATIONAL WAX TECHNICIAN PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety
 University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety 1.0 Introduction... 1 1.1 Purpose... 1 1.2 Regulatory Background...
University of Wisconsin-Madison Hazard Communication Standard Policy Dept. of Environment, Health & Safety Office of Chemical Safety 1.0 Introduction... 1 1.1 Purpose... 1 1.2 Regulatory Background...
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN
 NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
NATIONAL ELECTROLOGY PRACTICAL EXAMINATION CANDIDATE INFORMATION BULLETIN Please visit www.nictesting.org for the most current bulletin prior to testing. This bulletin contains important information regarding
APPROVAL REVIEW PROCEDURES
 Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
UNIVERSITY OF CALIFORNIA SANTA BARBARA Medical Waste Management Plan Large Quantity Generator with Onsite Treatment 417
 UNIVERSITY OF CALIFORNIA SANTA BARBARA Large Quantity Generator with Onsite Treatment 417 Contents I. Introduction II. Definitions III. Responsibilities IV. Types and Quantity of Medical Waste Generated
UNIVERSITY OF CALIFORNIA SANTA BARBARA Large Quantity Generator with Onsite Treatment 417 Contents I. Introduction II. Definitions III. Responsibilities IV. Types and Quantity of Medical Waste Generated
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
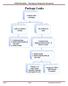 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
PORTAGE COUNTY COMBINED GENERAL HEALTH DISTRICT ENVIRONMENTAL DIVISION 2017 NEW BODY ART ESTABLISHMENT PERMIT TO OPERATE APPLICATION INSTRUCTIONS
 PORTAGE COUNTY COMBINED GENERAL HEALTH DISTRICT ENVIRONMENTAL DIVISION 2017 NEW BODY ART ESTABLISHMENT PERMIT TO OPERATE APPLICATION INSTRUCTIONS Ohio Administrative Code (OAC) 3701-9-02(A) In accordance
PORTAGE COUNTY COMBINED GENERAL HEALTH DISTRICT ENVIRONMENTAL DIVISION 2017 NEW BODY ART ESTABLISHMENT PERMIT TO OPERATE APPLICATION INSTRUCTIONS Ohio Administrative Code (OAC) 3701-9-02(A) In accordance
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions
 Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Texas Department of Licensing & Regulation Health & Safety Sanitation Standards Topic Definitions 83.100 Health & Safety Definitions Clarity for licensee on health, safety & sanitation responsibilities
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS
 PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
under Council Medical Devices Directive (93/42/EEC) as updated directive 2007/47/EEC
 Page 1 of 7 under Council Medical Devices Directive (93/42/EEC) as updated directive 2007/47/EEC New Miana Pura East, Roras Road, Sialkot - Pakistan Tel: +92-52-3560135 Fax: +92-52-3563647 E-mail: info@longstoneintl.com
Page 1 of 7 under Council Medical Devices Directive (93/42/EEC) as updated directive 2007/47/EEC New Miana Pura East, Roras Road, Sialkot - Pakistan Tel: +92-52-3560135 Fax: +92-52-3563647 E-mail: info@longstoneintl.com
Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid
 Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid Purpose: This Safe Method of Use applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs),
Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid Purpose: This Safe Method of Use applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs),
SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS
 SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS Purpose To ensure the safe disposal of potentially contaminated sharps, syringes, clothing and any other waste products. Scope
SHARPS MANAGEMENT AND DISPOSAL OF SHARPS, SYRINGES & CONTAMINATED PRODUCTS Purpose To ensure the safe disposal of potentially contaminated sharps, syringes, clothing and any other waste products. Scope
INFECTION PREVENTION AND CONTROL SAFE USE AND DISPOSAL OF SHARPS
 INFECTION PREVENTION AND CONTROL SAFE USE AND DISPOSAL OF SHARPS Policy title Safe Use and Disposal of Sharps Infection Control and Prevention (IPC) Policy CL05C reference Policy category Clinical Relevant
INFECTION PREVENTION AND CONTROL SAFE USE AND DISPOSAL OF SHARPS Policy title Safe Use and Disposal of Sharps Infection Control and Prevention (IPC) Policy CL05C reference Policy category Clinical Relevant
RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER SANITARY REQUIREMENTS TABLE OF CONTENTS
 RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER 0200-03 SANITARY REQUIREMENTS TABLE OF CONTENTS 0200-03-.01 Applicability 0200-03-.02 Violations 0200-03-.03 Location 0200-03-.04 Communicable
RULES OF TENNESSEE BOARD OF COSMETOLOGY AND BARBER EXAMINERS CHAPTER 0200-03 SANITARY REQUIREMENTS TABLE OF CONTENTS 0200-03-.01 Applicability 0200-03-.02 Violations 0200-03-.03 Location 0200-03-.04 Communicable
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
BODY ART ESTABLISHMENT INTRODUCTION GUIDE
 BODY ART ESTABLISHMENT INTRODUCTION GUIDE Toledo-Lucas County Health Department 635 N. Erie Street Toledo, OH 43604 Phone: (419) 213-4100 ext. 3 Fax: (419) 213-4141 INTRODUCTION This guide has been developed
BODY ART ESTABLISHMENT INTRODUCTION GUIDE Toledo-Lucas County Health Department 635 N. Erie Street Toledo, OH 43604 Phone: (419) 213-4100 ext. 3 Fax: (419) 213-4141 INTRODUCTION This guide has been developed
SKIDMORE COLLEGE. Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS. Introduction -- Pg. 2
 SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
UPEI Waste Disposal Protocol
 UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
(c) BODY ART ESTABLISHMENT means any location, whether temporary or permanent, where the practices of body art are performed.
 DEPARTMENT OF PUBLIC HEALTH AND ENVIRONMENT Division of Environmental Health and Sustainability BODY ART ESTABLISHMENTS 6 CCR 1010-22 [Editor s Notes follow the text of the rules at the end of this CCR
DEPARTMENT OF PUBLIC HEALTH AND ENVIRONMENT Division of Environmental Health and Sustainability BODY ART ESTABLISHMENTS 6 CCR 1010-22 [Editor s Notes follow the text of the rules at the end of this CCR
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
Steps Towards Integrating Your IBC and Your IACUC. By Brenda Wong UCSD Biosafety Officer June 25, 2009
 Steps Towards Integrating Your IBC and Your IACUC By Brenda Wong UCSD Biosafety Officer June 25, 2009 UCSD Background School of Medicine Cancer Center 2 Hospitals Scripps Institution of Oceanography School
Steps Towards Integrating Your IBC and Your IACUC By Brenda Wong UCSD Biosafety Officer June 25, 2009 UCSD Background School of Medicine Cancer Center 2 Hospitals Scripps Institution of Oceanography School
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
SANITARY REQUIREMENTS FOR TATTOO & BODY PIERCING ESTABLISHMENTS
 SANITARY REQUIREMENTS FOR TATTOO & BODY PIERCING ESTABLISHMENTS A REGULATION OF THE BOARD OF HEALTH OF THE MAHONING COUNTY GENERAL HEALTH DISTRICT ESTABLISHING REGISTRATION REQUIREMENTS FOR TATTOO & BODY
SANITARY REQUIREMENTS FOR TATTOO & BODY PIERCING ESTABLISHMENTS A REGULATION OF THE BOARD OF HEALTH OF THE MAHONING COUNTY GENERAL HEALTH DISTRICT ESTABLISHING REGISTRATION REQUIREMENTS FOR TATTOO & BODY
City and County of Denver Rules and Regulations for Body Artist, Body Art Establishments, and Mobile Body Art Vehicles Chapter 24 DRMC
 City and County of Denver Rules and Regulations for Body Artist Body Art Establishments and Mobile Body Art Vehicles Chapter 24 DRMC Adopted by the Board of Environmental Health on March 11 1999 And Amended
City and County of Denver Rules and Regulations for Body Artist Body Art Establishments and Mobile Body Art Vehicles Chapter 24 DRMC Adopted by the Board of Environmental Health on March 11 1999 And Amended
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
