MEDICAL WASTE MANAGEMENT
|
|
|
- Ashlee Townsend
- 6 years ago
- Views:
Transcription
1 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste. Duke University must manage these types of waste in order to minimize potential personnel exposures and to assure environmentally sound disposal of medical waste. RESPONSIBILITIES Departments shall ensure that all medical waste generators within their department comply with this policy. Generators of medical waste shall: Minimize the amount of waste generated. Dispose of wastes according to this Duke Policy. Packagers of regulated medical waste for offsite treatment will: Complete training to comply with packaging and labeling requirements set by The Department of Transportation (DOT). Medical Center Environmental Services (EVS: ) is responsible for supplying and disposing of the sharps containers in patient areas and medical center. Duke Regional Hospital DRH uses an eco friendly reusable sharps management system. Daniels reusable Sharpsmart system is serviced by a contractor several times a week. Contact the Supply Chain Material s Supervisor ( ) regarding service issues. OESO shall: Assist in the identification and classification of medical waste. Assist in developing the appropriate procedures to handle and dispose of medical waste in compliance with both North Carolina and other applicable waste management laws or rules, where needed. PROCEDURES Regulated medical waste requires special packaging, labeling, and must be decontaminated prior to disposal. According the North Carolina Rules and Duke Occupational and Environmental Safety Office, categories of regulated waste include:
2 1. Liquid or semi liquid human blood, human blood components and products made from human blood > 20 ml in volume. 2. Liquid volumes of human body fluids including semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid >20 ml in volume. 3. Contaminated items that would release blood or body fluids in a liquid state when compressed, such as soaked surgical sponges. 4. Sharps, including needles, syringes with attached needles, slides, and cover slips, capillary tubes, Pasteur pipets, or scalpel blades. 5. Pathological Waste includes human tissues, organs and body parts; and the carcasses and body parts of all animals that were known to have been exposed to pathogens that are potentially dangerous to humans during research, were used in the production of biologicals or in vivo testing of pharmaceuticals, or that died of a known or suspected disease transmissible to humans. 6. Microbiological Waste includes cultures and stocks of infectious agents. At Duke, this also includes recombinant DNA/transgenic organisms. Specific waste handling practices will vary depending on the individual type of medical waste being generated, and consequently, general guidelines for a variety of different work areas are presented. AREA SPECIFIC MANAGEMENT PRACTICES Inpatient Areas pleurevacs are placed in the biohazard containers located in the soiled equipment rooms. The biohazard containers are shipped for offsite treatment and disposal. Medical Center Environmental Services (EVS: ) is responsible for the distribution and collection of these containers. Urine and feces may be carefully poured down the sanitary sewer (commode). Sharps including needles, syringes with attached needles, slides and cover slips, or scalpel blades are placed in the puncture resistant sharps containers (needle boxes) located in every patient room, the medication carts, and the code blue carts. To prevent needlestick injuries, needles must not be recapped, purposely bent or broken by hand, removed from disposable syringes or otherwise manipulated by hand. Sharps containers are closed when two thirds filled. EVS is responsible for supplying and disposing of the needle boxes in patient areas. If a box is found to be two thirds full, it will be picked up and replaced. Needle boxes are packaged in biohazard boxes and shipped for offsite treatment and disposal. Solid waste from patient rooms (used gloves, masks, disposable gowns, gauze, etc.) are not regulated medical waste and need not be decontaminated prior to disposal as routine trash; however they must be sufficiently contained in leakproof bags for transport and disposal. Operating Rooms
3 pleurevacs are placed in the leakproof bags in the operating room. The bags are placed in biohazard containers. Medical Center Environmental Services (EVS: ) is responsible for the distribution, collection, and disposal of these containers. Contaminated items that would release blood or body fluids in a liquid state when compressed (soaked surgical sponges) are also placed in the biohazard containers in the operating room. As the biohazard containers in each operating room are filled, they are placed in the hallway for pick up by EVS for offsite treatment and disposal. EVS will routinely inspect these areas and remove waste accordingly. Sharps are placed in the puncture resistant containers (needle boxes) in each operating room. To prevent needlestick injuries, needles must not be recapped, purposely bent or broken by hand, removed from disposable syringes or otherwise manipulated by hand. Sharps containers are closed when two thirds filled. EVS responsible for the distribution, collection, and disposal of these containers. Solid waste from the operating rooms (disposable gloves, masks, disposable gowns, gauze, etc.) other than that above are not regulated medical waste and need not be decontaminated prior to disposal as routine trash; however, they must be sufficiently contained in leak proof bags for transport and disposal. On Site Clinics pleurevacs, as well as trace chemotherapy waste are placed in the biohazard containers located in the utility rooms of the clinics. Medical Center Environmental Services (EVS: ) is responsible for the distribution, collection, and disposal of these containers. Urine and feces may be carefully poured down the sanitary sewer (commode). Sharps are placed in the puncture resistant containers (needle boxes) in areas of use throughout the clinics. To prevent needlestick injuries, needles must not be recapped, purposely bent or broken by hand, removed from disposable syringes or otherwise manipulated by hand. Sharps containers are closed when two thirds filled. EVS responsible for the distribution, collection, and disposal of these containers. Solid waste from patient rooms (disposable gloves, mask, disposable gowns, gauze, etc.) is not regulated medical waste and need not be decontaminated prior to disposal as routine trash; however, they must be sufficiently contained in leakproof bags for transport and disposal. Off Site Clinics pleurevacs, as well as trace chemotherapy, are placed in the biohazard containers located in the utility rooms in the off site clinics. Use a Duke preferred vendor for distribution and collection of these containers. If you do not yet have your site set up with the vendor, contact Duke Procurement
4 ( ) for more information. Biological Safety Urine and feces may be carefully poured down the sanitary sewer (commode). Sharps are placed in the puncture resistant containers (needle boxes) in areas of use throughout the clinics. To prevent needlestick injuries, needles must not be recapped, purposely bent or broken by hand, removed from disposable syringes or otherwise manipulated by hand. Sharps containers are closed when two thirds filled and collected by a Duke preferred vendor for disposal. Solid waste from patient rooms (disposable gloves, mask, disposable gowns, gauze, etc.) are not regulated medical waste and need not be decontaminated prior to disposal as routine trash; however, they must be sufficiently contained in leakproof bags for transport and disposal. Clinical Laboratories Biological/Microbiological wastes (patient specimens, cultures, or stocks of etiologic agents) are placed in biohazard bags and collected by Medical Center Environmental Services (EVS: ). The waste is then packaged appropriately in biohazard shipping containers that are shipped for offsite treatment and disposal. At BSL3, waste must be steam sterilized (autoclaved 90 minutes at 250 o F (121 o C), 15 psi) onsite before it is collected for disposal. Urine may be carefully poured down the sanitary sewer. Sharps are placed in the puncture resistant containers (needle boxes) in areas of use throughout the clinics. To prevent needlestick injuries, needles must not be recapped, purposely bent or broken by hand, removed from disposable syringes or otherwise manipulated by hand. Sharps containers are closed when two thirds filled. EVS responsible for the distribution, collection, and disposal of these containers. All anatomical/pathological human waste must be incinerated. Such wastes are placed in a leak resistant biohazard container, submitted to EVS ( ) and shipped for offsite treatment and disposal. Solid wastes from laboratories (disposable gloves, gauze, etc.) are not regulated medical waste and need not be decontaminated prior to disposal as routine trash; however, they must be sufficiently contained in leakproof bags for transport and disposal. On or Off Site Research (BSL1 BSL2) / Teaching Laboratories (BSL1 BSL2) There are two options for solid waste treatment. Option 1: Off site treatment is set up through a preferred vendor and purchased/scheduled through your departmental business office. Some Duke Medical Center buildings are already set up through EVS ( ) for this service. Contact your department business office to determine. With this option, your department pays the vendor or EVS to deliver supplies of biohazard bags and biohazard sharps containers (needle boxes).
5 Option 2: On site treatment is performed using steam sterilization via autoclaving for *90 minutes at 250 o F (121 o C), 15 psi. ALL autoclavable bags are contained within a secondary autoclavable bin/pan to contain leaks within the autoclave. Do not pour heated agarose down the drain. Autoclaved bags are transported within a leak proof secondary container (trash can, cart, bin, etc.) to the buildings outside trash dumpster. With this option, your department purchases autoclavable biohazard bags and sharps containers as done with other lab supplies. *NC Management Rules (15A NCAC 13B Section.1200) allows for steam sterilization for 45 minutes at 250F 15 psi ONLY if the laboratory monitors the operations of the autoclave unit under condition of full loading for effectiveness of treatment. Monitoring shall be performed NO LESS THAN ONCE PER WEEK through the use of biological indicators. A log of each test of effectiveness of treatment performed shall be maintained and shall include the type of indicator used, date, time, and result of test. If the indicator shows failure of the effectiveness of treatment, a change in the way you load the waste may be necessary or the autoclave may need to be taken out of service for maintenance. You must create a standard operating procedure for this method to address failures. Biological/Microbiological wastes (human blood products <20 ml, cultures or stocks of etiologic agents) are collected in autoclavable bags and treated using Option 1 or 2. Additionally, chemical disinfection is a treatment option for liquid biological waste. See Laboratory Safety Manual, Waste Management under The Biological Safety Section for more information. All anatomical/pathological human waste must be incinerated. Such wastes are placed in a leak resistant biohazard container, submitted to the vendor or to EVS , and using Option 1, shipped for offsite treatment and disposal. All animal carcasses are coordinated for proper treatment through Duke Lab Animal Resources (DLAR: ). If carcasses are in a chemical fixative, contact OESO Environmental Programs ( ) for advice on disposal. Urine may be carefully poured down the sanitary sewer. Sharps, including needles, syringes with attached needles, slides, and cover slips, capillary tubes, Pasteur pipets, or scalpel blades are placed in the puncture resistant sharps containers (needle boxes) located in areas of use throughout the laboratory. To prevent needlestick injuries, needles must not be recapped, purposely bent or broken by hand, removed from disposable syringes by or otherwise manipulated by hand. Sharps containers are closed when two thirds filled. They are sent off site for treatment as noted in Option 1 or placed in an autoclavable bag for autoclaving on site as noted in option 2. If performing Option 2, the autoclaved bags are safely transported within secondary containment to the buildings dumpster for disposal. Serological Pipets can puncture bags and therefore are bundled separately in an appropriate puncture resistant biohazard labeled box that is lined with an autoclavable bag. The box is taped closed and treated using Option 1 or Option 2 above. Solid waste from laboratories (disposable gloves, gauze, etc.) are not regulated medical waste and need not be decontaminated prior to disposal as regular trash; however, they must be sufficiently contained in leakproof bags for transport and disposal. Pharmacies
6 Pharmacy waste (i.e. sharps) is not regulated medical waste. Refer to the Hazardous Drugs Policy and the Pharmaceutical Waste FAQ. REFERENCES North Carolina Administrative Code (NCAC) 10G , General Statute (GS) 130A , Effective Sept. 1, 1990.
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
Medical Waste Management Plan
 Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Medical Waste Management Plan Safety Services - Biosafety University of California, Davis Version 1.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website:
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Safe Handling and Disposal of Sharps. Reference Guide
 Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
Safe Handling and Disposal of Sharps Reference Guide Safe Handling and Disposal of Syringes and other Sharps All staff involved in the administration of a drug or other substance should be trained in the
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
Spring 2005 Pollution Prevention Workshop For Healthcare
 Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
Spring 2005 Pollution Prevention Workshop For Healthcare Regulated Medical Waste Compliance Issues Daniel Salzler ADEQ Solid Waste Inspection & Compliance Unit Arizona Solid Waste Rules Arizona Administrative
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS
 PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
PLAN REVIEW APPLICATION PACKET BODY ART ESTABLISHMENTS Greene County Public Health 360 Wilson Drive Xenia, OH 45385 (937) 374-5600 / (937) 374-5607 www.gcph.info Submit completed plan review packet, Infection
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
UPEI Waste Disposal Protocol
 UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
UPEI Waste Disposal Protocol Purpose: The purpose of this document is to ensure that waste is disposed of properly and safely in order to ensure the safety of all who handle waste. Waste Pretreatment:
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
MEDICAL WASTE MANAGEMENT PLAN
 MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
MEDICAL WASTE MANAGEMENT PLAN University of California, Davis Version 2.0 Main Office: 276 Hoagland Hall, Davis, CA, 95616 Phone: (530) 752-1493 Fax: (530) 752-4527 Website: safetyservices.ucdavis.edu
Medical Waste Manual. California State University, Chico
 California State University, Chico The Department of Environmental Health and Safety March 2017 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal of Biohazardous
California State University, Chico The Department of Environmental Health and Safety March 2017 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal of Biohazardous
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Medical Waste Manual. California State University, Chico
 California State University, Chico The Department of Environmental Health and Safety Revised January 4, 2011 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal
California State University, Chico The Department of Environmental Health and Safety Revised January 4, 2011 TABLE OF CONTENTS Section Page 1.0 Introduction... 1-1 2.0 Containment, Storage, and Disposal
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
UNIVERSITY OF CALIFORNIA SANTA BARBARA Medical Waste Management Plan Large Quantity Generator with Onsite Treatment 417
 UNIVERSITY OF CALIFORNIA SANTA BARBARA Large Quantity Generator with Onsite Treatment 417 Contents I. Introduction II. Definitions III. Responsibilities IV. Types and Quantity of Medical Waste Generated
UNIVERSITY OF CALIFORNIA SANTA BARBARA Large Quantity Generator with Onsite Treatment 417 Contents I. Introduction II. Definitions III. Responsibilities IV. Types and Quantity of Medical Waste Generated
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN
 BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection Prevention
SKIDMORE COLLEGE. Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS. Introduction -- Pg. 2
 SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
SKIDMORE COLLEGE Biohazardous Waste Management Policy and Exposure Control Plan TABLE OF CONTENTS Introduction -- Pg. 2 Glossary of Terms -- Pg. 3-4 I. Identification/Definition of Biohazardous and Regulated
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
ATS-SOI-5731 Page: 1 of 5. Approval Block. Prepared by: Signature Date Margaret Crouse 18 JUN Reviewed by: Signature Date
 ATS-SOI-5731 Page: 1 of 5 Approval Block Prepared by: Signature Date Margaret Crouse 18 JUN 2014 Reviewed by: Signature Date Brian Flynn 18 JUN 2014 Approved by: Signature Date Kristal Jewell 18 JUN 2014
ATS-SOI-5731 Page: 1 of 5 Approval Block Prepared by: Signature Date Margaret Crouse 18 JUN 2014 Reviewed by: Signature Date Brian Flynn 18 JUN 2014 Approved by: Signature Date Kristal Jewell 18 JUN 2014
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
Original Date:
 Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Title: Sharps Safety Index Number: (Func. - Categ. - Sr.No.) Function: Facility Management and Safety Category: Safety Scope of application: All Departments/Units/ Sections Original Date: 06.08.2008 Next
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Infectious Waste Contingency Plan
 Infectious Waste Contingency Plan Office of Environmental Health and Safety September 2016 Table of Contents I. Introduction..3 II.Facility Identification and Contact Information..3 III. Emergency Contacts...3-4
Infectious Waste Contingency Plan Office of Environmental Health and Safety September 2016 Table of Contents I. Introduction..3 II.Facility Identification and Contact Information..3 III. Emergency Contacts...3-4
METHODS OF IMPLEMENTATION AND CONTROL
 Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Universal Precautions: METHODS OF IMPLEMENTATION AND CONTROL All employees will utilize universal precautions (MIOSHA Rule 325.70005) Rule 5. Universal precautions shall be observed to prevent contact
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
KERN HEALTH SYSTEMS POLICIES AND PROCEDURES 2.21-P
 Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
Page 1 of5 RESPONSIBLE DEPARTMENT HEAD: Director of Quality Improvement, Health Education and Disease Management Review Date 08/29/97 08/00 01102 08/2005 OS/2010 Effective Date 09/01103 11118/05 DS/;ZOhtJ
APPROVAL REVIEW PROCEDURES
 Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
Summit County Public Health 1867 West Market Street Akron, Ohio 44313 Phone: (330) 923-4891 Toll-free: 1 (877) 687-0002 Fax: (330) 923-6436 www.scphoh.org APPROVAL REVIEW PROCEDURES Ohio Law requires that
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
Infection Prevention Guidelines. Safe Use, Handling & Disposal of Sharps
 Infection Prevention Guidelines Safe Use, Handling & Disposal of Sharps Author: Anne Tateson Owner: Infection Prevention Team Publisher: Infection Prevention Date of Version Issue: February 2015 Version:
Infection Prevention Guidelines Safe Use, Handling & Disposal of Sharps Author: Anne Tateson Owner: Infection Prevention Team Publisher: Infection Prevention Date of Version Issue: February 2015 Version:
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES
 BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
BODY ART /PIERCING PLAN REVIEW APPLICATION AND GUIDELINES Plan Review Request for a Body Art/Piercing Establishment Instructions 1. Complete the form and attached requested information in plan review packet.
BD Disposal Solutions Product Catalog
 Product Catalog Recognizing that human health and a healthy environment are inseparable Sharps Pharmaceutical Chemotherapy RCRA Accessories Driven by Sustainability We re working smarter to evolve BD Sharps
Product Catalog Recognizing that human health and a healthy environment are inseparable Sharps Pharmaceutical Chemotherapy RCRA Accessories Driven by Sustainability We re working smarter to evolve BD Sharps
BODY ART ESTABLISHMENT PLANNING APPLICATION
 BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN
 ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
ILLINOIS STATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN Revised 11/10/2017 Table of Contents 1. PURPOSE AND SCOPE... 2 2. RESPONSIBILITIES... 2 a. ENVIRONMENTAL HEALTH AND SAFETY... 2 b. STUDENT
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
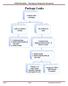 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Safe Sharps Disposal. Learn how to safely dispose of used sharps including needles, lancets and syringes. Expanded Syringe Access Program
 Safe Sharps Disposal Learn how to safely dispose of used sharps including needles, lancets and syringes. Expanded Syringe Access Program How to Safely Dispose of Household Sharps Millions of people use
Safe Sharps Disposal Learn how to safely dispose of used sharps including needles, lancets and syringes. Expanded Syringe Access Program How to Safely Dispose of Household Sharps Millions of people use
What is infection control?
 Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
Infection control What is infection control? It is the discipline concerned with preventing healthcareassociated infection. It is an essential part of the infrastructure of health care. Standard principles
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT
 SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
SUTTER COUNTY DEVELOPMENT SERVICES DEPARTMENT Building Inspection Planning Fire Services Road Maintenance Code Enforcement Environmental Health Engineering Water Resources SUMMARY OF THE SAFE BODY ART
Environmental Standard Operating Procedure (ESOP)
 Environmental Standard Operating Procedure (ESOP) Originating Office: Natural Resources Environmental Affairs (NREA) Office File Name: MWO-ESOP Revision: 24 January 2017 Prepared By: Subject Matter Expert
Environmental Standard Operating Procedure (ESOP) Originating Office: Natural Resources Environmental Affairs (NREA) Office File Name: MWO-ESOP Revision: 24 January 2017 Prepared By: Subject Matter Expert
LAPORTE COUNTY TATTOO & BODY PIERCING ORDINANCE
 LAPORTE COUNTY TATTOO & BODY PIERCING ORDINANCE 2011-07 1 Ordinance No. 2011-07 OF THE BOARD OF COMMISSIONERS OF LAPORTE COUNTY, INDIANA Whereas, the Indiana State Department of Health has promulgated
LAPORTE COUNTY TATTOO & BODY PIERCING ORDINANCE 2011-07 1 Ordinance No. 2011-07 OF THE BOARD OF COMMISSIONERS OF LAPORTE COUNTY, INDIANA Whereas, the Indiana State Department of Health has promulgated
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
PREVENT. Sharps Containers. If you are not completely satisfied with any McKesson Brands product, you may return it for a full refund or credit.
 Sharps Containers McKesson offers a full line of sharps containers and accessories that feature OSHA-compliant safety features and excellent performance. The line was created to help protect our customers
Sharps Containers McKesson offers a full line of sharps containers and accessories that feature OSHA-compliant safety features and excellent performance. The line was created to help protect our customers
University of Nevada, Reno Operational Plan for Management of Biohazardous Waste
 University of Nevada, Reno Operational Plan for Management of Biohazardous Waste Scope This operational plan covers management of biohazardous waste produced as a result of University of Nevada, Reno (UNR)
University of Nevada, Reno Operational Plan for Management of Biohazardous Waste Scope This operational plan covers management of biohazardous waste produced as a result of University of Nevada, Reno (UNR)
Queen's University Technicians Position Description Questionnaire. Immediate Supervisor: Manager, Biohazard, Radiation and Chemical Safety
 Queen's University Technicians Position Description Questionnaire Field of Work: Safety Technician (Biohazard and Chemical Safety) Name: TBA Department: Environmental Health & Safety Date: June 22, 2018
Queen's University Technicians Position Description Questionnaire Field of Work: Safety Technician (Biohazard and Chemical Safety) Name: TBA Department: Environmental Health & Safety Date: June 22, 2018
