Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan
|
|
|
- Coral Roberts
- 5 years ago
- Views:
Transcription
1 Cooper Union Kanbar Center for Biomedical Engineering Laboratory Safety Plan (last revised Aug 30, 2011) The Maurice Kanbar Center for Biomedical Engineering Room 704 NAB David Wootton, Ph.D. Director Office: Room 714 NAB Phone: x307 (on campus) David Orbach, MS, MD, Assistant Director Office: Room 206 NAB Phone: X4042 (on campus) Cell (347) Dionne Lutz, BS, EdM, Lab Technician Office: Room 704 NAB Phone: X326 (on campus) Adapted from: Harvard Environmental Health and Safety Biosafety Office
2 TABLE OF CONTENTS 1. Introduction 2. General Lab Guidelines 3. Personal Protective and Safety Equipment i. Eye Protection ii. Gloves iii. Lab Coats iv. Eyewash v. Shower vi. Fire Extinguishers vii. Biosafety Cabinet 4. Biosafety Levels 5. Laboratory Work Practices i. Biosafety Level 1 ii. Biosafety Level 2 iii. Biosafety Level 3 & 4 6. Physical Containment Checklist i. Biosafety Level 1 ii. Biosafety Level 2 iii. Biosafety Level 3 & 4 7. Use of Sharps in the Laboratory i. Guidelines for Sharps Use ii. Broken Glassware Disposal 8. Use of Chemicals in the laboratory i. MSDS ii. Chemical Procurement iii. Chemical Storage iv. Chemical Handling 9. Waste Disposal i. General Waste ii. Chemical Waste Disposal iii. Solid Biohazardous Waste Disposal iv. Liquid Waste Disposal v. Sharps Disposal vi. Mixed Waste Disposal 10. Commonly Used Disinfectants i. Lysol ii. Chlorine Bleach iii. Alcohols 11. Spills i. Chemical Spills ii. Biological Spills 12. Bloodborne Pathogens 13. Universal Precautions 14. Incompatible Chemicals
3 INTRODUCTION Procedures and facilities involved in protecting laboratory workers and the general public from laboratory biological hazards are governed by federal, state and local regulations. Some of these rules have the force of law while others are simply guidelines. Many granting agencies require grantees to certify that they adhere to both the suggested federal guidelines and the legally mandated requirements. All labs working with human blood, tissue, and body fluids must adhere to the OSHA standard. The NIH and the Center for Disease Control (CDC) publish a set of guidelines for work with infectious organisms entitled "Biosafety in Microbiological and Biomedical Laboratories" (HHS Publication # CDC ). The publication is available online. GENERAL LAB GUIDELINES Common Sense Rules: No eating, drinking, smoking, gum chewing, or make-up application is allowed in the biomedical engineering labs. Refrigerators/Freezers in the lab are not for storage of food or drink (see above) Avoid behavior which may distract another worker (no horseplay) Avoid unnecessary exposure to hazardous chemicals Wash your hands frequently and before leaving the lab. Avoid working alone in the lab and do not work alone if the procedure being conducted is hazardous Lab Cleanliness: The worksite is to be maintained in a clean and sanitary condition. Plastic-backed paper may be used to cover benches, but it should be removed and replaced when contaminated and at the end of the workday. Work surfaces should be washed and disinfected at the end of an experiment, at the close of the day, and after a spill. Reusable items are to be decontaminated before washing. Clean laboratory glassware after use. Equipment Use: No unattended or unsupervised use of mechanical equipment is permitted. Do not attempt to use equipment that you have not been properly trained to use. Limit the number of visitors to the BME labs. Visitors are not permitted to use lab equipment or supplies. Please direct anyone interested in using lab equipment or supplies to Drs. Wootton or Orbach Apparel & Personal Protective Equipment: Eye protection should be used (goggles, face shield, etc) during handling of chemicals or BSL2 materials Gloves should be worn to minimize skin exposure to chemicals Lab coats are available as are disposable covers. Do not wear lab coats or gloves out of the lab!! Confine long hair and loose clothing
4 No open toe shoes PERSONAL PROTECTIVE & SAFETY EQUIPMENT Eye protection Eye protection is required during the use of chemicals and other potentially hazardous conditions. Goggles or safety glasses which protect the eye from splashing or splattering of chemicals and infectious agents will be provided. Face shields must be worn during the use of corrosive (acids) and caustic chemicals. Safety goggles should be cleaned with ethanol after each use. Corrective eyewear does not provide adequate laboratory protection and may not be used as a substitute for eye protection. Contact lens application or removal is not permitted in the laboratory. Since they are permeable to certain vapors, it is suggested that contact wearers obtain special safety goggles which do not have air vents. Visitors to the lab must comply with the same eye protection policy required of students and employees. Gloves Gloves are an important part of personal protection. Various types of gloves are available for specific laboratory hazards. During use of bio-hazardous materials, toxic substances and other hazardous conditions, the use of gloves is mandatory. Prior to use, gloves should be inspected for perforations, discoloration, and tears. Gloves protect hands from chemical spills and contamination and should be removed and appropriately disposed of once contaminated or torn. Contamination with bio-hazardous materials requires that they be disposed of in red hazardous waste containers. Exposure to solvents and detergents may result in dermatitis, skin burns, irritation or sensitization. If you are unsure which glove is appropriate for your application, please consult Drs. Wootton or Orbach. Gloves contaminated with bio-hazardous materials must not be re-used. They must also be removed prior to leaving the immediate work area to ensure against contamination of shared use areas. Proper removal of latex gloves is outlined in the following procedure: Grasp the outside of glove with the opposite gloved hand Peel the glove off Hold removed glove in gloved hand Slide fingers of ungloved hand under remaining glove at wrist Peel glove off over first glove Gloves should be inside out with one inside the other Discard gloves in appropriate waste container
5 Lab coats Adopted from Lab coats, gowns, and aprons are to be worn to protect the skin from spilled chemicals or biohazardous materials. The materials utilized to manufacture these protective barriers either works by absorbing or deflecting the spill. They should be worn when handling any biological or chemical substance Lab coats or aprons are worn to absorb or deflect spills and prevent corrosive or toxic substances from reaching the skin. Which is used is largely a matter of personal preference, but one or the other should be available to every individual working in a laboratory. The stockroom provides a basic cotton blend coat; however, individuals may choose to order coats of Tyvek, a spun, bonded polyester material made by DuPont, which is the best (most impermeable) material for these garments. Eyewash An eyewash is to be used in emergency conditions where a hazardous substance has come into contact with the face and/or eyes. It is used to irrigate or flush the eyes and face of the hazardous substance. It should be located within 10 seconds of the chemical use area and access must be unobstructed. Below, you will find instructions on the proper eyewash use. Shower: Located out the door to the left in the hallway of the 7 th floor. Safety showers are used in emergency situations to prevent injury from hazardous substances. There are a number of uses for safety showers including dilution, cooling, irrigation, and extinguishing. Chemicals which come in contact with skin or clothing can be diluted to nontoxic or non-harmful levels. Chemical exposure may also cause elevated skin temperature which can be cooled and flushed by a deluge shower. In conditions where clothing catches fire, the safety shower may be used to extinguish the flames. The first 10 to 15 seconds after exposure to a hazardous or corrosive chemical are the most important; therefore use of a safety shower should not be delayed. Once the shower is in operation, the affected party must remove equipment and clothing under the shower. Below, you will find instructions on the proper emergency shower use. Proper Use of Eyewash and Emergency Shower Equipment: In case of chemical exposure, flush skin or eyes with cool water for at least 15 minutes. DO NOT RUB! Get medical assistance immediately following flushing. If possible, continue flushing while on way to medical help. (any contact lens solution is also a portable source of flushing even if expired) Know the effects of chemicals with which you are working. Read, ask questions about, and understand material safety data sheets for each chemical with which you work. Always wear personal protective equipment. Learn the location and use of all emergency equipment, even if you are working in a new area for only a brief time. (Fire extinguishers located at each entrance to lab.) Know how to help others reach showers or eyewashes and how to help them get medical assistance. Hold your eyes open with your hands while using an eyewash to be sure water reaches the eyes.
6 Remove contaminated clothing after the shower has been activated. Immediately wash off even small amounts of chemicals. Fire Extinguishers The number, type and placement of fire extinguishers is regulated by the New York City Fire Department (FDNY). In general fire extinguishers are useful for small fires in an emergency situation. Use of fire extinguishers should be restricted to faculty or staff and in cases where the fire is small, the appropriate class of extinguisher is available, and the user has been trained in the use of fire extinguishers. Fire extinguishers are serviced on a regular basis to confirm amount of extinguishing material. Cooper Union s official policy consists of calling the fire department in the event of a fire. There are five basic classes of fire extinguishers; the classifications refer to the types of burning materials they can extinguish. The rating system consists of A, B, C, D and K Class fire extinguishers. Class A and B fire extinguishers have an additional rating system consisting of a number placed in front of the classification rating. (i.e.: 2-A). The numerical rating determines the extinguishing effectiveness (capacity) for each size and type of extinguisher. Class A: Extinguishers are used on ordinary combustible materials, including wood, paper, cloth, rubber, and many plastics. The numerical rating associated with this type of extinguisher refers to the amount of water capacity of the fire extinguisher and amount of fire that can be extinguished. Class B: Extinguishers are used on fires involving flammable and combustible liquids, such as grease, oil, gasoline, alcohol, oil-based paints, lacquers, etc. The numerical rating associated with this type of extinguisher approximates the amount of area the fire covers in square feet Class C: Extinguishers are used to extinguish electrically energized equipment or fires. There is no numerical rating associated with this type of extinguisher. Extinguishers with the C rating indicate that the extinguishing material is non-conductive Class D: Extinguishers with a D rating are for fires involving combustible metals, such as magnesium, titanium, and sodium. These extinguishers do not have a rating and are often specific for the type of metal used.
7 Class K: Extinguishers for fires involving vegetable and animal oils, or fats in cooking appliances. These types of extinguishers are typically used in commercial kitchens, of restaurants, cafeterias, and caterers. Along with the five basic fire extinguisher types, there are multi-use extinguishers. These consist of AB, BC, and ABC rated fire extinguishers. AB: This multipurpose extinguisher contains AFF Foam and is used on both A and B class fires. BC: This multipurpose extinguisher is a regular type dry chemical extinguisher. It is filled with sodium bicarbonate or potassium bicarbonate. Residue left by this type of extinguisher is mildly corrosive and must be cleaned to prevent damage to materials ABC: This is the multipurpose dry chemical extinguisher. The ABC type is filled with monoammonium phosphate, a yellow powder that leaves a sticky residue that may be damaging to electrical appliances such as computers. (ref: Biosafety cabinet Biosafety cabinets (or "tissue culture hoods") protect both you and your work from contamination. The barrier between the work and worker is a curtain of sterile air descending from the top of the cabinet after passing through a HEPA filter. Air flow is balanced so that some air is taken from the room and, along with sterile cabinet air, sucked into a horizontal grill at the front of the work surface. Don't confuse a biosafety cabinet with the "clean air bench. A clean bench has no front screen - air from the work surface blows at you. Clean benches should not be used for work with microorganisms and potentially infected cells. There are several types of biosafety cabinets. Most researchers use Type II cabinets - ones
8 that re-circulate a fraction of the air through a HEPA filter back into the workspace. The biosafety cabinet in Room704NABis Type II. A biosafety cabinet must have regular maintenance and certification by a professional to assure that it protects you, your experiments, and the environment. Each cabinet should be certified when it is installed, each time it is moved or repaired, and at least once each year. (Last certified ~Nov 2009) Tips for using a biosafety cabinet: Before beginning work, decontaminate the work surface with alcohol Run the biosafety cabinet for a minimum of 15 minutes before starting work. Keep the front and back grilles clear. Minimize hand and arm movement in and out of the cabinet. Make sure the sash is at the proper height (not too low, not too high).
9 BIOSAFETY LEVELS The four biosafety levels summarized in the table below are a convenient classification system for describing physical containment and work practices needed for different types of work. The Biomedical Engineering laboratories at Cooper Union are currently Biosafety Level 1. No work at biosafety Level 2 can be done without contacting Dr. Wootton and Dr. Ahmed, Cooper Union s safety officer. Biosafety level 3 and 4 work is not permitted at any time at Cooper Union. SUMMARY OF BIOSAFETY LEVELS Biosafety Level Risk (Individual / Community) Practices & Techniques Safety Equipment Examples 1 low/low Standard Microbiological Practices. 2 moderate/low Level 1 plus: lab coats, limited access, biohazard signs on doors & equipment. 3 high/moderate Level 2 plus: special protective clothing, controlled access through entrance room, biological waste autoclaved; preferably in facility. 4 high/high Level 3 plus: entrance through change room. Complete change of clothing from street to lab-oratory gear, shower at exit. Wastes decontaminated on exit from facility. None: primary containment provided by adherence to standard lab practices during open bench operations. Partial containment (i.e., Class I or II biosafety cabinets for procedures which produce aerosols) Partial containment equipment used for all manipulations of infectious materials, directional airflow. Maximum containment (i.e., Class III cabinet or partial containment in combination with fullbody, air-supplied positive-pressure personnel suit) used for all procedures & activities. E. Coli K12, most nonprimate mammalian tissue & cell lines. Hepatitis B, Salmonella typi, human tumor cell lines, lymphoid lines carrying inducible EBV, many common human pathogens. Yellow fever, M. tuberculosis, Short term culture of tissue from non-human primates until cultures are known to be free of Herpesvirus simiae (B. virus) Ebola & Marburg Virus Propagation of Herpes virus simiae (monkey B virus).
10 LABORATORY WORK PRACTICES: Biosafety Level 1 (Standard Work Practices) Wash hands after handling biologicals, after taking off gloves, and before leaving the lab. Decontaminate work surface daily and after spills. No eating, drinking, or smoking in the lab. Use mechanical pipetting devices. If you wear contact lenses, consider wearing goggles or a face shield while working. Avoid aerosol formation. Place all solid biological waste in red bags for proper disposal Liquids must be disinfected before sink disposal. Control insect and rodent infestation Biosafety Level 2: All biosafety level 1 practices Use biological safety cabinets to contain aerosol-producing procedures. Use of centrifuges with sealed heads or safety cups is recommended. Wear protective clothing including a lab coat or protective gown, goggles or face shield (if splashes are possible) and gloves. Leave protective clothing behind in the lab when you leave. Change the gloves frequently. Restrict access to the lab. Staff must receive annual training in safety procedures appropriate to the organisms being studied. Offer immunization and/or tests for the agents being used (Hepatitis vaccinations, TB tests). Accidental exposures must be reported to the laboratory director so that medical evaluation and treatment can be provided. Changes in procedures or equipment are evaluated. Use leak proof primary and secondary containers when transporting infectious materials Biosafety Level 3& 4 (not applicable to work done at Cooper Union)
11 PHYSICAL CONTAINMENT CHECK LIST Biosafety Level 1 (A Basic Laboratory) Sink for washing hands. Lab designed for easy cleaning (no rugs!) Non-porous, alkali, acid and solvent resistant benchtops. Screens on windows if they open. Spaces between walls and equipment must be accessible for cleaning Biosafety Level 2 Biosafety 1 facility requirements A door sign with: Universal Biohazard symbol, listing the organisms in use and the name and phone number of the laboratory director. The sign should indicate any special requirements for entering the lab (gowns, goggles,...). If there is likelihood of aerosol generation Biosafety Cabinets (Class II) should be installed and certified annually. Eye wash and safety shower. Each laboratory must have biosafety manual. A method for decontaminating wastes must be available. Autoclaves, chemical disinfectants or an incinerator may be appropriate. Biosafety Level 3& 4 (not applicable to work done at Cooper Union) USE OF SHARPS IN THE LABORATORY Most serious biological accidents are caused by puncture wounds. Objects that can puncture skin are called "sharps" and are given special treatment in every laboratory. Of course punctures are possible with pencils, paper clips, etc, but biosafety rules restrict themselves to laboratory items. Examples abound: hypodermic needles, glass Pasteur pipettes, razor blades, broken glass, suture needles... The best way to avoid sharps injury is to avoid using sharps. Substitute plastic when possible. Plastic transfer pipettes may be a good replacement for Pasteur pipettes. Plasticware can eliminate broken glass problems. Self sheathing needles are used when the work involves blood collection. There are two aspects to dealing with sharps: using them and throwing them away. Both can be risky and require special care. Guidelines for Sharps Use Avoid using needles and syringes whenever possible. Do not bend, break, or otherwise manipulate needles. Do not remove scalpel blades by hand. Do not recap needles. Do not remove needles from syringes. Throw away the entire syringe-needle combination!! Be careful cleaning up after procedures that require the use of syringes and needles. Sharp items may have become hidden in the garbage.
12 Guidelines for Sharps Disposal (see waste disposal below) Broken Glassware Disposal: Broken glass is not to be picked up by hand; use forceps or tweezers. Place clean broken glassware into the standard broken glassware cardboard boxes. If the glassware is contaminated, disinfect it before disposal. Contaminated broken test tubes or other small items of broken glassware should be placed directly into red Sharps containers. USE OF CHEMICALS IN THE LABORATORY Material Safety Data Sheets (MSDS) For each hazardous chemical used in the lab there is a MSDS readily available. The MSDS can be found in bright yellow binders located just outside thelaboratory. Prior to using any chemical in the lab, the corresponding MSDS should be reviewed. Each MSDS contains the following information: Chemical identity Physical and chemical characteristics Physical hazards Health hazards Routes of exposure Permissible exposure limits Whether the chemical is listed on the National Toxicology Program annual report on carcinogens Precautions for safe handling and use Control measures such as engineering controls, work practices and personal protective equipment Emergency and first aid procedures Date of preparation or last change Name, address, & phone number of party responsible for preparing the MSDS Procurement Chemicals can only be ordered and brought into the lab by Cooper Union faculty and staff. It is important to verify that the amount ordered does not cause the lab to exceed storage limitations (see below). Before a substance is received, information on proper handling, storage and disposal should be obtained. Potential sources of this information are: MSDS, product data sheets (often can be found on supplier s website). When the new chemical is received, it 1) must be added to the chemical inventory posted in each lab and 2) the MSDS must be filed in the two yellow binders. Anyone who violates these policies may lose access to the lab. Storage Containers In general, chemicals should be stored in their original containers with labels intact. If a chemical is transferred to another container for storage, the container must be labeled with the
13 following information: chemical identity, appropriate hazards warnings, concentration of solution (if applicable), name of person who transferred the chemical to the container, and date of transfer. Amounts The amount of each type of chemical that can be stored in the lab is limited by the Occupational Health and Safety Administration (OSHA) and the NYC fire department. The table below summarizes the maximum laboratory storage limits. In addition, avoid storing excess amounts of chemicals or chemicals which are no longer in use. Chemical Storage Limits Lab Type Fire Rating Fire Protection Flammable Liquids Flammable Solids Oxidizing Materials Unstable Reactive I 2 hours Sprinklers 30 gals 15 lbs 50 lbs 12 lbs II 1 hour Sprinklers 25 gals 10 lbs 40 lbs 6 lbs III 2 hours No Sprinklers 20 gals 6 lbs 30 lbs 3 lbs IV 1 hour No Sprinklers 15 gals 3 lbs 20 lbs 2 lbs Handling Appropriate engineering controls and personal protective equipment as indicated by MSDS should be used while handling chemicals. In the event of exposure or emergency, safety showers, eyewash stations, and fire extinguishers are available in or near each lab (see above). In general, try to minimize use and exposure to hazardous chemicals. Any container used (even temporarily) must be labeled with the following information: chemical identity, concentration of chemical (if applicable), name/initials of person who is using the chemical in the container, and date of use. When chemicals are hand carried between labs, a secondary container must be used. Remember, do not wear gloves outside of the lab. The secondary container provides the necessary protection. Many chemical combinations are incompatible; mixing incompatible chemicals can create an explosion or a poisonous gas. Check the incompatible chemicals table in chapter 14 prior to mixing chemicals. Store incompatible chemicals separately. Disposal (see below) WASTE DISPOSAL Many different types of waste are generated in the laboratory. Please use the definitions provided below to classify all the waste you generate in the lab as: general, chemical, solid biohazardous, liquid biohazardous, sharp, or mixed. Dispose each type of waste separately using the procedures outlined below.
14 1. General Waste definition: General waste contains materials which are free of biologic, hazardous chemical, or radioactive (does not apply for BME at Cooper Union) contamination. Some examples are: paper, boxes, Styrofoam, etc. procedure: This type of waste can be discarded in normal trash cans located in the lab. Note: bovine knees purchased from the butcher fall in the category of general waste. However, please do not put large bones into the general waste container. Double bag the remnants (using standard black garbage bags) and bring the waste directly to the garbage cans in the loading dock. 2. Chemical Waste Disposal definition: Waste containing chemical substances e.g., laboratory chemicals, empty bottles of lab or pharmacy chemicals, disinfectants that have expired or are no longer needed, etc. procedure: Chemical waste disposal is handled by Ms Victoria Hinz, the designated Hazardous Waste Disposal Coordinator and Chemistry Stockroom Technician. (212) (located in the chemical stock room; she will call Leard Environmental Services, Inc.) Ms. Hinz schedules hazardous waste disposal for the Engineering School and periodically sends announcements regarding the dates of hazardous waste collection. Waste to be disposed is to be labeled with an accurate description of the container contents and approximate amount. In accordance with the Federal Cradle to Grave requirements, all hazardous wastes (including not only chemical reagents, radioactives and biological materials but also lubricants, oils, batteries, flammable sprays and cleaning solutions) must be disposed of by coordination with Mr. Torres, and may not be disposed of through any third party contractor not approved by Buildings and Grounds. (ref: 3. Solid Biohazardous Waste Disposal definition: Any solid contaminated with medical or biohazardous waste that does not go into the sharps container. This includes empty specimen containers, all surgical gloves, culture dishes used for or containing cell cultures, articles contaminated with small volumes liquid biohazardous waste (not enough to drip), and animal tissue and parts. procedure: Solid biohazardous waste should be collected in a rigid, leak-proof, container labeled with the universal biohazard symbol and lined with 2 red plastic biohazard bag. There are two biohazardous garbage cans in the biomedical engineering lab for this purpose. Bags containing BL2 waste must be treated first with disinfectant (see Section?) and then discarded in the waste. Please notify Lab Technician Dionne Lutz, or Dr. Wootton or Dr. Orbach when the waste container is full so that a medical waste pick-up can be arranged. Animal tissue and parts are to be contained in the freezer until the morning of the medical waste pick-up, transferred to a red plastic biohazard bag and then placed in one of the medical waste cardboard boxes. 3. Liquid Biohazardous Waste Disposal definition :Any liquid contaminated with medical or biohazardous waste, including human and animal blood. Any potentially infectious bodily fluids or body substances. procedure: All liquid biohazardous waste must be decontaminated by chemical disinfection. Add chlorine bleach to the biohazardous waste container in which liquid waste is accumulated
15 such that the final bleach dilution is 1/10 of the final volume of liquid waste. Allow a least 20 minutes for the disinfectant to act. After decontamination, the liquid can be discarded in the sink. The drain should be flushed with water after the waste (now disinfected) is poured into the drain. The container, if disposable, should be placed in the red biohazardous garbage. 5. Sharps Disposal definition: Objects or devices having acute rigid corner, edges, or protrusions capable of cutting or piercing. Some examples are: needles, scalpel blades, razor blades, Pasteur pipettes, etc. procedures: Place into standard "Sharps" containers. These are thick plastic containers with a biohazard symbol. Throw away the entire syringe-needle combination!! Do not overfill the Sharps containers. Close them when they are 3/4 full. Discard closed sharps containers by placing in red biohazardous bag and box for medical waste pick-up. 6. Mixed Waste Disposal Biohazardous/Chemical Waste The approach to Biological wastes containing hazardous or potentially hazardous chemicals is similar to radioactive biologicals: Destroy the infectious agents with a chemical disinfectant and dispose of the results as chemical waste. Be careful in your choice of disinfectants. Some disinfectants, such as chlorine bleach, can react with the chemical to form an unpleasant surprise. Check with Environmental Health and Safety ( ) to be sure. COMMONLY USED DISINFECTANTS Lysol Lysol concentrate is a general purpose household disinfectant. This antibacterial all purpose cleaner kills Salmonella, E. Coli, Staph, and other bacteria. It meets the Federal Food Code Requirements for a no rinse sanitizer and is a degreaser and deodorizer. In the proportion 1:128, it can be used for light-duty cleaning and sanitizing and for heavy-duty cleaning and sanitizing in the proportion 1:46. It is recommended for use on floors, walls, counters, and equipment and in Foodservice and dietary areas, dairies, food processing plants, supermarkets, and schools. It also controls mold and mildew. Ref: Chlorine Bleach Household Clorox is a 5.25% solution of sodium hypochlorite. A 1/10 dilution will inactivate most microorganisms in 20 minutes. Some bacteria and most spores are more resistant. Mycobacterium needs a 1/5 dilution for inactivation. The concentration needed to decontaminate depends on the organic load of the material to be treated. Dilute bleach solutions decompose at room temperature and should be made up frequently. A 1/10 solution of household bleach remains useful for no more than a month. Routine practice is to prepare a fresh 10% solution of bleach weekly.
16 Alcohols Ethyl alcohol and isopropyl alcohol diluted to 70% in water are useful for surface decontamination. Alcohols are non-corrosive and are appropriate for decontamination of materials that can be damaged by halogens. Alcohols should be used with care. Avoid the temptation to use them at 100%. It should be recalled that a 100% alcohol solution is an excellent desiccant. Desiccation will often preserve, rather than kill, many microorganisms. Some organisms, such as Mycobacterium, are not inactivated by 70% ethanol. Remember that alcohol compounds burn. Do not use with fire or flame. SPILLS Chemical Spills There is a chemical spill kit located in the Biomedical Engineering lab. Biological Spills Keep a spill kit handy. Basic equipment is a concentrated disinfectant (chlorine bleach or Lysol) a package of paper towels, household rubber gloves, and forceps to pick up broken glass. Spill in a Biological Safety Cabinet (BSL 1 & 2) Leave the cabinet turned on While wearing gloves, spray or wipe cabinet walls, work surfaces, and equipment with disinfectant. If necessary, flood the work surface, as well as drain pans and catch basins below the work surface, with disinfectant (usually 1/10 Clorox) for at least 20 minutes contact time. Soak up the disinfectant and spill with paper towels. Drain the catch basin into a container. Lift front exhaust grill and tray, and wipe all surfaces. Ensure that no paper towels or solid debris are blown into the area beneath the grill. Wash hands and exposed skin areas with disinfectant. Dr. Wootton should be notified if the spill overflows into the interior of the cabinet. It may be necessary to do a more extensive cabinet decontamination. Small (<500 ml) spill of outside of a safety cabinet (BSL 1 & 2) Wearing gloves and a labcoat, cover the spill with paper towels and disinfectant (usually a 1/10 dilution of bleach) Allow sufficient contact time with disinfectant (usually >20 minutes) Pick up towels and discard into biohazard waste container. Pick up broken glass with forceps and place in Sharps container. Re-wipe the spill area with disinfectant and wash your hands with soap or handwashing disinfectant. Large spill of BL1 material outside of a safety cabinet (>500 ml) GET HELP from a professor or trained staff member! Wearing household gloves and a labcoat, absorb spill with paper towels. Using a detergent solution, clean the spill site. Wipe down the spill site with paper towels soaked in a disinfectant such as chlorine bleach, diluted 1/10.
17 Discard all contaminated materials in a biohazard waste container. Wash your hands with soap or handwashing disinfectant. Large spill of BL2 material outside of a safety cabinet (>500 ml) GET HELP from a professor or trained staff member! Keep people out of the area to prevent spread of the contamination. Post sign. Remove any contaminated clothing and put it into a biohazard bag for decontamination later. Wash hands and exposed skin and inform your supervisor about the spill. Put on protective clothing (lab coat, gloves and, if indicated, face protection and shoe covers) and assemble clean-up materials (disinfectant, autoclavable container or bag, forceps and paper towels). Pick up any broken glass with forceps and dispose of it in Sharps container. Ring the spill with disinfectant and mix it into the spill. Take care not to over-dilute the disinfectant. After at least 20 minutes contact time, clean-up liquids, and re-wipe the spill area with disinfectant. Collect all contaminated materials for decontamination and wash your hands with soap or handwashing disinfectant. Emergency Procedures for Major and Minor Chemical Spills Major Chemical Spills Evacuate the immediate area Activate the Fire Alarm Pull Station (Fire Alarm Pull Stations are located by every staircase in all buildings) Call the following and state your name, location, chemical(s) involved, and the amount spilled: o New York State Department of Environmental Conservation (NYSDEC) Emergency 24-Hour Spill Hotline (800) o o Leard Environmental Services, Inc. (888) Scott Leard (631) Scott Leard (pager) Buildings and Grounds Ext. 160 After Hours Contact Maintenance Personnel on duty (through security guard) Alan Wolf Campus-Wide Safety Coordinator, Cellular: (917) , office (212) Jody Grapes, Campus-Wide Safety Officer (212) office Simon Ben-Avi, Campus Emergency Coordinator, Dean of Engineering: (917) Attend to any person who may have been contaminated Wait in a safe area for the response team Do not allow unauthorized personnel to enter the contaminated area
18 Report the incident to your supervisor and the Buildings and Ground office (x161). Fill out Accident/Incident Report. Send the original report to the Personnel Department in the Business Office and send a copy of the report to the Buildings and Grounds Office. Minor Chemical Spills Stop, Think, and Carefully Plan Cleanup o Refer to Material Safety Data Sheet (MSDS) to determine the correct cleanup procedure for the material spilled. Call Victoria Heinz in the chemical stock room (212) who will call Leard Environmental Services, Inc. If malodorous/hazardous vapors are generated from the chemical spill which can spread outside the local areas, leave the area and then call the New York State Department of Environmental Conservation If flammable, eliminate any ignition sources as soon as possible (Gas Emergency Shut-off Valve) BLOODBORNE PATHOGENS Definition Bloodborne pathogens are microorganisms which are capable of causing disease that and are present in human blood. Bloodborne pathogens can also be found in other body fluids including: semen, breast milk, saliva, urine, and tears. Bloodborne Pathogens & Cooper Union Currently there is no research done at Cooper Union involving fluids, tissue, or cells from human origin. However, the following material is presented for informational purposes. History In response to reports of health workers becoming infected with HIV and hepatitis B virus (HBV) in 1990 the federal government intervened to tighten work procedures and workers rights. The agency involved, OSHA, is a part of the Labor Department. OSHA's interests emphasize worker safety. Since most people at risk are in the healthcare professions, most of the regulations are designed around issues confronting patient care. However, there is substantial effort to protect workers in other fields. Thus, laboratory workers are covered. As a result of hearings and expert panel suggestions OSHA published a Bloodborne Pathogen Standard in December The Standard has the force of law and must be obeyed by any institution working with blood or blood products. For a government regulation the standard is remarkably short and surprisingly readable. A copy can be obtained by visiting: Who's Covered? Coverage includes all employees who may encounter biological materials that might carry a bloodborne pathogen while performing routine job duties. These materials include but are not limited to blood, serum or plasma, semen, vaginal secretions, cerebrospinal fluid, and other body fluids that have been contaminated with blood. Researchers using unfixed tissue, organs, primary
19 human cell cultures and related culture medium are also covered by the Standard. Pathogens Covered In addition to Human Immunodeficiency Viruses (HIV-1, HIV-2) and Hepatitis B Virus (HBV) the standard covers a wide variety of bloodborne diseases. Some of these are simian immunodeficiency virus (SIV), and the biological agents that cause syphilis, malaria, babesiosis, brucellosis, leptospirosis, arboviral infections, relapsing fever, Creutzfeldt-Jacob disease, viral hemorrhagic fever, and human T-lymphotropic virus type I. What the Standard Requires from the Employer Write an exposure control plan. It explains: bloodborne pathogens. universal precautions. equipment to protect employee. HBV vaccination. exposure follow-up. decontamination. List jobs in which employees can become exposed. Train employees in those jobs annually. Keep records of who has been trained. Keep records of any exposures. Offer HBV vaccination to people in listed jobs. Provide employees with safety equipment (gowns, gloves, masks, face shields...). What the Standard Requires from the Employee Follow Universal Precautions and good laboratory practices (see next page). Decide whether to have an HBV vaccination. Be sure to maintain skills and knowledge through annual training and other avenues. Report exposures immediately. UNIVERSAL PRECAUTIONS The concept of "Universal Precaution" arises from the fact that one can never be absolutely certain that a sample comes from a disease free sources. It is prudent to consider biological specimens of human origin (see below for list) as contaminated and to act accordingly. Bloodborne Disease Transmission Bloodborne disease transmission requires the virus enter the recipient's general blood circulation. This can be through direct blood-to-blood transmission (transfusions) or indirect (dirty needles). Less obvious routes of transmission are through skin breaks and via the mucous membranes of the eye, nose, mouth... It should be appreciated that skin breaks at risk can be simple dermatitis, acne, cuts, abrasions or hangnails. Materials to be handled using universal precautions All human blood, blood products, certain body fluids (semen, vaginal, cerebrospinal, synovial, pleural, peritoneal, pericardial, and amniotic), any body fluids in which visible blood is present, and any unfixed human tissue or organ.
20 Recommended personal protective equipment to prevent exposure: Disposable gloves that are changed as soon as they become contaminated. Masks, eye protection, face shields -worn whenever splashes, spray and/or droplets may come into contact with the mucous membranes. Lab coats, gowns, aprons - to protect skin surfaces and street clothing. Universal precaution work practices that prevent exposure: Eating, drinking, smoking, applying cosmetics or lip balm, handling contact lenses, etc. are prohibited in work areas. Food is not stored in work areas. Mouth pipetting is prohibited. Wash hands whenever gloves are changed and before leaving the work area. Personal Protective Equipment is to be removed before leaving the work area. Signs and labels The biohazard symbol must be on containers of infectious waste, and on refrigerators and other equipment where blood and other potentially infectious materials are stored. Hospitals The following is a list of nearby hospitals and their emergency room numbers where students or employees may be sent in the case of a medical emergency. Many medical emergencies will require an ambulance, in which case you should dial 911 for emergency assistance. * Beth Israel Medical Center 1 st Avenue & 16 th St. (212) Cabrini Medical Center 227 E. 19 th St. (212) New York Eye and Ear Infirmary 310 E 14ths ST. Eye Trauma Center (212)
BSL-2 Emergency Plan
 BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
BSL-2 Emergency Plan Spills General Spill Cleanup Guidelines: Know how to get the HVAC unit servicing the lab space shut down in order to limit the spread of contamination. Wear gloves and lab coat. Use
Biological Safety Training
 Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
Biological Safety Training Introduction to Biological Safety Biological Hazards are divided into 4 Biosafety Levels BSL 1 BSL 2 BSL 3 BSL4 Biosafety levels define the lab requirements, protective clothing,
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN
 FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
FLORIDA GULF COAST UNIVERSITY DEPARTMENT OF REHABILITATION SCIENCES BIOSAFETY AND INFECTIOUS AGENTS CONTROL PLAN PURPOSE: This policy establishes minimum requirements for the handling, storage and disposal
Safety Office -- Laboratory Inspection Form
 RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
RESEARCH DIVISION Safety Office -- Laboratory Inspection Form NOTES: Satisfactory laboratory inspection is required prior to initiation of research New inspection required if Biosafety Level changes Annual
Roosevelt Biosafety Training. Created 10/2015
 Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Roosevelt Biosafety Training Created 10/2015 Objectives Identify risks and hazards in biological laboratories Understand biosafety levels for laboratories and the proper procedures for working in them
Emergency Procedures Specific Biological Spill Clean-Up Guidelines
 Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
Emergency Procedures 3.1.1. Biological Spills Spill kit materials and written procedures shall be kept in each laboratory where work with microorganisms is conducted. Basic equipment includes concentrated
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
General Lab Safety Rules and Practices SOP-GLSRP-01
 Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Standard Operating Procedure General Lab Safety Rules and Practices SOP Number: SOP-GLSRP-01 Category: Lab Process Supersedes: N/A Effective Date: December 1, 2017 Pages 5 Subject: General Lab Safety Rules
Biohazardous Waste. 1. Solid Biohazardous Waste (non-sharps) Storage
 Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
Biohazardous Waste There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate
BSL2 Exposure Control Plan: Human or Non Human Primate Materials
 Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
Prepared/Revised by Tamara Casebolt, Ph.D Date 6/7/2017 Reviewed by Carolyn Keierleber, Ph.D Date 09/20/2017 A. Hazards Human blood or other primate cells and tissue have the potential to harbor infectious
The following standard practices, safety equipment, and facility requirements apply to BSL-1:
 Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Standard Microbiological Practices for Biosafety Level 1 Laboratories at the University of Tennessee-Knoxville, Institute of Agriculture and Graduate School of Medicine Overview and Definitions Standard
Bloodborne Pathogens Exposure Control Plan. December 2003
 Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Bloodborne Pathogens Exposure Control Plan December 2003 H://winfiles/safety/bloodborne pathogens/ofd Bloodborne Pathogens Plan.doc pg 2 PURPOSE: The purpose of this exposure control plan is to: 1. Eliminate
Provide a brief description of the procedure and infectious organisms used:
 Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Western Carolina University Standard Operating Procedure for the Safe Handling of Infectious Organisms at BSL-2 Containment Section 1. Contact Information Procedure Title: Procedure Author: Date of Creation/Revision:
Regulated Medical Waste. Be sure to sign in!
 Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
Regulated Medical Waste Be sure to sign in! Waste Management Training You must receive this training if you: Add regulated medical waste into an accumulation container Determine if a material is regulated
List any references used for the procedure design (research publications, etc.):
 Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Western Carolina University Standard Operating Procedure for the Safe Handling of Animals A-BSL2 Containment Section 1. Contact Information Procedure Author: Date of SOP Creation/Revision: Name of Responsible
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # Approved: 10/3/18
 Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Enhanced BSL2 (BSL2+) Lab Policy IBC Policy # 150.1 Approved: 10/3/18 DIRECTIONS: All lab members must review this policy and sign/date the confirmation page at the end. I. GENERAL INFORMATION A. Institutional
Safety Rules for Laboratory
 Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
Safety Rules for Laboratory These protocols are intended to protect you and make your laboratory experience enjoyable and productive. Section I: CVM General Laboratory Protocols (these rules apply to all
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT September 2010 Table of Contents Section Page Background 1 Definitions
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
Bloodborne Pathogens Exposure Control Plan Environmental Health, Safety, and Risk Management Department Box 6113, SFA Station Nacogdoches, Texas 75962-6113 January 2011 Revised May 2017 APPLICABILITY These
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET
 TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
TEN EASY STEPS FOR CLEANING A SPILL IN THE BIOSAFETY CABINET Ten Easy Steps for Cleaning a Spill in the Biosafety Cabinet For over 40 years, NuAire has been providing laboratory equipment that better enables
Brazosport College Life Science Laboratory Safety Rules and Regulations
 Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
Brazosport College Life Science Laboratory Safety Rules and Regulations Laboratory Safety Procedures for Biology Labs Permanent Link: http://bit.ly/bc-labsafety The risks incurred in the biology laboratories
University Of Florida. Bloodborne Pathogen Program. Standard Operating Procedures
 University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) BBP Standard Operating Procedures Page 1 of 13 University Of Florida Bloodborne
x. ANNUAL REVIEW SIGNATURE SHEET
 x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
x. ANNUAL REVIEW SIGNATURE SHEET PROCEDURE TITLE: UNIVERSAL PRECAUTIONS Signature on this page insures that each procedure has been reviewed annually. Any changes will be reflected on the procedure by
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION
 State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
State of Kuwait Ministry of Health Infection Control Directorate SAFE INJECTION May 2010 Contents I. Introduction II. Prevention strategies III. Best practices for injection A. General safety practices
Standard Operating Procedure for Biosafety Cabinet Use
 NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
NIPISSING UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY Standard Operating Procedure for Biosafety Cabinet Use PREPARED BY: DAVE VADNAIS JULY 27, 2016 R EVIEWED NOVEMBER 30, 2017 STANDARD OPERATING PROCEDURE
OSHA: Occupational Safety and Health Administration PPE Personal protective equipment
 Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Bloodborne Pathogens University of Tennessee Safety Program HM-010 Document Contact: EHS Date effective: March 15, 2011 Revision Date: October 2, 2017 Purpose The purpose of this written program is to
Laboratory Orientation. Biological Screening
 Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
Laboratory Orientation Laboratory Orientation Safety Clean technique Reagent preparation Use of basic equipment Quality assurance : Laboratory Orientation 2 Safety National Forensic Science Technology
CCS Administrative Procedure T Biosafety for Laboratory Settings
 CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
CCS Administrative Procedure 2.30.05-T Biosafety for Laboratory Settings Implementing Board Policy 2.30.05 Contact: College Biosafety Hygiene Officers, (phone # to be determined) 1.0 Purpose Community
Package Leaks. OH&S Biosafety Emergency Response Document. Examine outer packaging. Leaks or evidence of leaks. No evidence of leaks
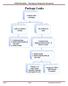 Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
Package Leaks Examine outer packaging Leaks or evidence of leaks No evidence of leaks 1. Contain package 2. Notify UAB Biosafety @ 934-2487 3. Notify Sender Examine inner pkging/contents in BSC - if there
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab
 The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
The Aim Of Biosafety Training Is To Increase Your Ability To Recognize And Reduce Hazards In a BSL1 Lab Think before you do anything What could possibly happen? What is the worst thing that could happen?
Disposal of Biohazard Wastes
 4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
4.24.1 POLICY Exceptions Radioactive Materials Administrators and principal investigators (PIs) are responsible for ensuring that biohazard wastes generated by University units are collected and disposed
TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee
 Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
Page 1 of 5 TEMPLE UNIVERSITY - Research Administration Institutional Biosafety Committee STANDARD OPERATING PROCEDURE SOP# 1.0 BIOSAFETY LEVEL 1 (BSL1) PROCEDURES A. Purpose This standard operating procedure
SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-002 FOR SHARPS USAGE AND DISPOSAL SCOPE This policy
Disposal of Biological Waste
 Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
Disposal of Biological Waste Biological Waste Disposal / Supplies Biological Waste Boxes Available in designated areas of research buildings (consult EH&S, Department administrator, other researchers)
A ppendix 15 WUStL Bloodborne Pathogens Exposure Control Plan Research Laboratory-Specific Work Practices
 Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Specifc Work Practices Check List for Principal Investigators and Laboratory M anagers Discuss with staff tasks that involve handling of potentially infectious materials and how to perform such tasks in
Infection Control 101
 Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Infection Control 101 Infection Control Nosocomial vs. HAIs Standard Precautions/Body Substance Isolation (BSI) Protective environment to prevent HAIs PPE (latex precautions) Biohazard Waste Transmission-based
Deadly Bloodborne Diseases
 What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
What and Why This Refresher Blood Borne Pathogens on-line training is offered for all returning employees of Harnett County Schools who have previously completed the Initial BBP training video. This is
STANDARD: Laboratory Safety Effective: March 20, 2018
 University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
University of North Dakota Department of Medical Laboratory Science Grand Forks, ND STANDARD: Laboratory Safety Effective: March 20, 2018 PURPOSE This standard establishes general safe practices in the
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS
 Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Michigan State University Athletic Training Students BLOOD BORNE PATHOGENS AND UNIVERSAL PRECAUTIONS The following principles must be applied when employees are potentially exposed to bloodborne pathogens:
Appendix C. Infectious Waste Guidelines
 Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Appendix C. Infectious Waste Guidelines C.1 Infectious Waste Generation and Treatment, as required by Ohio Administrative Code (OAC) Section 3745-27, is registered with the Ohio Environmental Protection
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook
 Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens: Exposure In The Workplace Employee Handbook Introduction There s a danger in the workplace that s not even visible to the naked eye, yet it could change your life forever if you re
Bloodborne Pathogens
 Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
Bloodborne Pathogens This PowerPoint is designed to inform those who may be exposed to blood and other bodily functions how to prevent spreading, avoid exposure, and what to do if exposed to infectious
BIOLOGICAL SAFETY INSPECTION CHECKLIST
 BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
BIOLOGICAL SAFETY INSPECTION CHECKLIST Section A : Contact Information (Principle Investigator) Last Name: First Name: Extension: Department: Building: Room: Section B: Inspection Date of Inspection: Time
Standard Operating Procedures
 Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
Standard Operating Procedures (V1_4/7/16) Safe Working Practices for Leica Laser Micro Dissection Microscope Table of Contents I. General Information II. Facility Orientation and Training III. Startup
INFECTION PREVENTION AND CONTROL PLAN
 INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
INFECTION PREVENTION AND CONTROL PLAN FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: ( ) The owner, employees and practitioners of the above body art facility have developed
San Bernardino Valley College. Blood Borne Pathogens. Exposure Control Program
 San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
San Bernardino Valley College Blood Borne Pathogens Exposure Control Program December 7, 2009 I. PURPOSE The Blood Borne Pathogens Exposure Control Program (BBP) has been developed by San Bernardino Valley
GENERAL CHEMISTRY LABORATORY SAFETY
 GENERAL CHEMISTRY LABORATORY SAFETY 1. Read the experiment before coming to Keyes 405. The more prepared you are, the safer and more efficient you will be in lab. 2. Think about what you need to wear to
GENERAL CHEMISTRY LABORATORY SAFETY 1. Read the experiment before coming to Keyes 405. The more prepared you are, the safer and more efficient you will be in lab. 2. Think about what you need to wear to
Updated by S. McNew, March Deborah Jung Microbiology Preparation Technician
 Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Southeast Missouri State University PROTOCOL FOR SCIENCE EQUIPMENT USAGE AT REGIONAL CAMPUSES WITH EMPHASIS ON BS240/BS242 MICROORGANISMS AND THEIR HUMAN HOSTS Updated by S. McNew, March 2018 Personnel
Handling and Disposing of Needles
 Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Guidance Document UBC-RMS-OHS-GDL 14-008 Effective date: June 4, 2014 Review date: June 4, 2014 Supersedes: N/A 1. SCOPE Handling and Disposing of Needles This guidance document on Handling and Disposing
Emergency Response and Biohazard Exposure Control Plan IBC Approved: 10/3/18
 Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Institutional Biosafety Committee Emergency Response and IBC Approved: 10/3/18 Table of Contents I. PURPOSE... 3 II. DEFINITIONS... 3 III. RESPONSIBILTIES... 4 IV. BIOHAZARDOUS SPILL EMERGENCY PREPAREDNESSS...
Standard Operating Procedures
 Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
Standard Operating Procedures Laboratory Specific Chemical: Formaldehyde Please fill out the form completely. Print a copy and insert into your Chemical Hygiene Plan. Department: Date when SOP was written:
PUBLIC HEALTH DEPARTMENT
 ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
ROBIN HODGKIN, M.P.A. Director STEPHEN W. MUNDAY, M.D., M.S. Health Officer COUNTY OF IMPERIAL PUBLIC HEALTH DEPARTMENT DIVISION OF ENVIRONMENTAL HEALTH 797 Main Street, Ste. B El Centro, CA 92243 Phone
Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text.
 Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
Click here to enter text. Laboratory Biosafety Manual Building/Lab Room No(s): Biosafety Containment level: BSL Click here to enter text. Date: Click here to enter text. Expires One year from the above
UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach
 Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Procedure #: UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach IBC-001 Date Adopted: October 10, 2017 Last Updated: Prepared By: Casey Webster, Research Compliance Administrator Reviewed
Biosafety Self-Audit Checklist
 Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
Biosafety Self-Audit Checklist Principal Investigator: Biosafety Certificate #: Location: Audited By: Date: Posting: Dalhousie University Hazard Identification poster with biohazard symbol posted on lab
CLEANING, SANITIZING, AND DISINFECTING
 CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
CLEANING, SANITIZING, AND DISINFECTING This section provides general information about cleaning, sanitizing, and disinfecting; guidelines for specific items commonly used in childcare and school settings;
REQUEST FOR QUOTE. Community Initiatives Bureau. Biohazardous Cleaning Service
 REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
REQUEST FOR QUOTE Community Initiatives Bureau Biohazardous Cleaning Service December 7, 2017 OVERVIEW The Boston Public Health Commission (BPHC) protects the health protects the health of Bostonians and
Annual Associate Safety Module. Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan
 Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
Annual Associate Safety Module Blood & Body Fluids: How To Prevent Exposure Your Exposure Control Plan Since you work in a healthcare facility, you may have potential exposure to blood or body fluids.
LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard
 ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
ERI Safety Videos Videos for Safety Meetings 2963 LABORATORY SAFETY SERIES: The OSHA Formaldehyde Standard Leader s Guide Marcom Group Ltd. INTRODUCTION TO THE PROGRAM Structure and Organization Information
ECU Radiation, Biosafety and Hazardous Substances Committee
 Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Standard Operating Procedure (SOP) Title (Samples Collected from Internal and External Agencies/Institutions) Note: As the infectious status of a patient s sample is unknown, precautions against exposure
Case Western Reserve University Department of Environmental Health & Safety
 Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
Case Western Reserve University Department of Environmental Health & Safety Laboratory Specific Supplement: CWRU Exposure Control Plan for Biohazards (including Bloodborne Pathogens) All laboratories at
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL. Table of Contents
 EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
EASTERN KENTUCKY UNIVERSITY HAZARD COMMUNICATION PROGRAM SUMMARY COMPLIANCE MANUAL Table of Contents I. OVERVIEW OF THE HAZARD COMMUNICATION STANDARD A. Background and Scope.................................
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
Bloodborne Pathogens Exposure Control Plan Document History Version Date Comments 0.2 January, 2018 Program Review Foreword This written program is site specific to UVa Facilities Management and is in
PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS
 PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS CESafety 1 WHMIS CESafety 2 What is WHMIS? WHMIS is a Canada-wide system designed to give employers and workers information about hazardous materials
PRESENTS WHMIS AND THE SAFE HANDLING OF HAZARDOUS MATERIALS CESafety 1 WHMIS CESafety 2 What is WHMIS? WHMIS is a Canada-wide system designed to give employers and workers information about hazardous materials
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy
 The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
The Management of Inoculation (Sharps) Injury or Blood Borne Pathogen Exposure Policy This policy applies to ALL sharps injuries where any hazardous substance (including, toxins, chemicals and human pathogens)
Management Plan for Employee Right-to-Know (ERK)
 Management Plan for Employee Right-to-Know (ERK) Table of Contents: Annual Review Form 1.0 Purpose 2.0 Globally Harmonized System (GHS) Compliance 3.0 Identification of Workplace Hazards 4.0 Hazard Assessments
Management Plan for Employee Right-to-Know (ERK) Table of Contents: Annual Review Form 1.0 Purpose 2.0 Globally Harmonized System (GHS) Compliance 3.0 Identification of Workplace Hazards 4.0 Hazard Assessments
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene
 Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Standard Microbiological Practices: Basic Biosafety Principles & Lab Hygiene Presented By: Biological Safety http://biosafety.utk.edu Training Overview: This training is designed to: Orient new personnel
Introduction. BSL Level 1-4 is also different from Risk Group 1-4 as described earlier but is very much related to each other.
 LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
LABORATORY BIOSAFETY CONTAINMENT LEVEL Introduction Laboratory Biosafety Containment Level or often known as Bio Safety Level (BSL) is referred to the containment level of the laboratory setting (including
GENERAL LAB SAFETY RULES. The following is a small list of the rules that MUST be followed in the lab!
 LABORATORY SAFETY GENERAL LAB SAFETY RULES The following is a small list of the rules that MUST be followed in the lab! General Lab Safety Rules: 1. Wear Goggles and Gloves when instructed. - Safety goggles
LABORATORY SAFETY GENERAL LAB SAFETY RULES The following is a small list of the rules that MUST be followed in the lab! General Lab Safety Rules: 1. Wear Goggles and Gloves when instructed. - Safety goggles
Sterilization A Training Module
 Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Sterilization A Training Module In This Training Module, You Will Learn: Definition of sterilization and disinfection What needs to be sterilized and disinfected What Personal Protective Equipment is required
Cleaning and Disinfection Protocol for Emergency Services Fire, Ambulance, Police, Search & Rescue
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College
 Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
Standard Operating Procedure for Blood Borne Infectious Disease Control Measures at Calvin College Clean up should be done by non-student employees and trained personnel only Cleaning Up BODY FLUIDS from
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Infection Control POLICY NUMBER: 860 Effective Date: August 31, 2006 SUBJECT: (INFECTIOUS) WASTE This cancels Nursing Procedure 860 dated
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
TARLETON STATE UNIVERSITY Biohazardous Waste Program Office of Risk Management and Safety June 2012 1. GENERAL The following information is provided to assist in developing requirements, guidelines and
Introductory Chemistry
 Introductory Chemistry Lab 1: Introduction and Safety Objectives Learn how work to safely in the chemical laboratory Learn when and how to use the safety equipment in the chemical laboratory Learn the
Introductory Chemistry Lab 1: Introduction and Safety Objectives Learn how work to safely in the chemical laboratory Learn when and how to use the safety equipment in the chemical laboratory Learn the
Instructor Guide. Georgia Department of Technical and Adult Education. Decontamination and Infection Control
 Instructor Guide Georgia Department of Technical and Adult Education Decontamination and Infection Control Copyright October 2002 by. All rights reserved. No part of this manual may be reproduced or transmitted
Instructor Guide Georgia Department of Technical and Adult Education Decontamination and Infection Control Copyright October 2002 by. All rights reserved. No part of this manual may be reproduced or transmitted
UNIVERSITY OF HAWAII Community Colleges ENVIRONMENTAL HEALTH AND SAFETY OFFICE HAZARD COMMUNICATION PROGRAM
 UNIVERSITY OF HAWAII Community Colleges ENVIRONMENTAL HEALTH AND SAFETY OFFICE HAZARD COMMUNICATION PROGRAM Revised January 2011 TABLE OF CONTENTS 1.0 INTRODUCTION 2.0 PROGRAM ADMINISTRATION 3.0 AN OVERVIEW
UNIVERSITY OF HAWAII Community Colleges ENVIRONMENTAL HEALTH AND SAFETY OFFICE HAZARD COMMUNICATION PROGRAM Revised January 2011 TABLE OF CONTENTS 1.0 INTRODUCTION 2.0 PROGRAM ADMINISTRATION 3.0 AN OVERVIEW
Working at Biosafety Level 2 (BSL-2)
 Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
Originator: 1.0 Purpose Department of Environmental Health and Safety The purpose of this document is to enhance safety at U of L by ensuring that everyone with potential exposure to infectious agents
2006 COURSE TITLE: HAZARDOUS MATERIALS SAFETY
 COURSE INTRODUCTION Hazardous substances are routinely used at work. However, that does not mean they are without risk. Some substances harm you right away. Others may cause health problems that show up
COURSE INTRODUCTION Hazardous substances are routinely used at work. However, that does not mean they are without risk. Some substances harm you right away. Others may cause health problems that show up
Material Safety Data Sheets (MSDS)
 Material Safety Data Sheets (MSDS) TIME: 20 minutes PURPOSE OF THE ACTIVITY: The participant will be able to read and use a Material Safety Data Sheet. MATERIALS NEEDED: q q Copies of the MSDS Sheets for
Material Safety Data Sheets (MSDS) TIME: 20 minutes PURPOSE OF THE ACTIVITY: The participant will be able to read and use a Material Safety Data Sheet. MATERIALS NEEDED: q q Copies of the MSDS Sheets for
Acid Or Alkali? Testing With Cabbage
 Acid Or Alkali? Testing With Cabbage Topic Using vegetables as an acid/base indicator Introduction Forensic scientists need to discover if someone has tampered with liquids (e.g., cosmetics, cleaning products,
Acid Or Alkali? Testing With Cabbage Topic Using vegetables as an acid/base indicator Introduction Forensic scientists need to discover if someone has tampered with liquids (e.g., cosmetics, cleaning products,
Welcome to the Hazard Communication Course
 Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Welcome to the Hazard Communication Course THE GLOSSARY A glossary is included in the Resources section on the home page of this course and on the OH&S website. These terms will be on the quiz. THE HAZARD
Biohazard Waste Management Plan
 WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
WAKE FOREST UNIVERSITY Biohazard Waste Management Plan Reynolda Campus WFU 1/9/2017 Questions or concerns regarding this plan should be directed to the Department of Environmental Health and Safety at
Self-Inspection 2018 Biosafety Containment Level 2 Requirements To be verified at an Inspection by Biohazard Committee Members
 Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
Self-nspection 2018 To be verified at an nspection by Biohazard Committee Members Containment requirements of the Canadian Biosafety Standard, 2 nd Edition, 2015, published by the Public Health gency of
VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers
 Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
Page 1 of 13 VGH Laboratory Guidelines Positive blood cultures from patients with suspect Ebola Virus Disease or other Viral Hemorrhagic Fevers Blood Culture technologist: 1. BACTEC FX signals positive
INFECTION PREVENTION AND CONTROL PLAN (IPCP)
 INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
INFECTION PREVENTION AND CONTROL PLAN (IPCP) FACILITY NAME: FACILITY ID: ADDRESS: CITY: STATE: ZIP: OWNER S NAME: PHONE: CONTACT PERSON: EMAIL: The owner, employees and practitioners of the above body
BODY ART ESTABLISHMENT PLANNING APPLICATION
 BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
BODY ART ESTABLISHMENT PLANNING APPLICATION Toledo-Lucas County Health Department 635 N. Erie Street Toledo-Lucas Toledo, County OH Health 43604 Phone: (419) 213-4100 Department ext. 3 Fax: (419) 213-4141
Product Name: Acne Medication Benzoyl Peroxide 10% Lotion Synonyms: None. Emergency telephone number: CHEMTREC
 1. Product and Company Identification Product Name: Acne Medication Benzoyl Peroxide 10% Lotion Synonyms: None Recommended Use: Uses Advised Against: No information available Supplier information: Garcoa
1. Product and Company Identification Product Name: Acne Medication Benzoyl Peroxide 10% Lotion Synonyms: None Recommended Use: Uses Advised Against: No information available Supplier information: Garcoa
Type of Application (Check One) New Protocol Revised Protocol Project Duration Start Date: End Date:
 Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Page 1 of 11 INSTITUTIONAL BIOSAFETY COMMITTEE Winston-Salem State University Application for the Use of Biohazardous Materials, Recombinant DNA and Infectious Agents 1. APPLICANT INFORMATION Assigned
Bloodborne Pathogens Safety in Your Workplace
 Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Bloodborne Pathogens Safety in Your Workplace COPYRIGHT NOTICE Copyright 2017 by Judy Adams. ALL RIGHTS RESERVED No part of this publication may be copied or distributed, transmitted, transcribed, stored
Chemistry Lab Safety Handout PSI Chemistry
 Chemistry Lab Safety Handout PSI Chemistry Name Chemistry is exciting! Each day in the laboratory you are given the opportunity to confront the unknown and to understand it. You can wonder how things work,
Chemistry Lab Safety Handout PSI Chemistry Name Chemistry is exciting! Each day in the laboratory you are given the opportunity to confront the unknown and to understand it. You can wonder how things work,
Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid
 Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid Purpose: This Safe Method of Use applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs),
Safe Method of Use for Hazardous Substances of Higher Risk 2 Hydrofluoric Acid Purpose: This Safe Method of Use applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs),
BODY ART FACILITY INFECTION PREVENTION AND CONTROL PLAN GUIDELINE
 Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Ventura County Environmental Health Division 800 S. Victoria Ave., Ventura CA 93009-1730 TELEPHONE: 805/654-5007 FAX: 805/477-1595 Internet Web Site Address: https://vcrma.org/body-art-program BODY ART
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation. Welcome to the Microbiology 22 Laboratory!
 Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
Mt. San Antonio College: Spring 2018 MICR 22 Lab Orientation Welcome to the Microbiology 22 Laboratory! Laboratory Objectives: To teach concepts of microbiological techniques using critically selected
BIOLOGICAL SAFETY MANUAL
 BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
BIOLOGICAL SAFETY MANUAL April, 2017 University of Northern Colorado Biological Safety Manual I. Introduction II. Definitions Human Blood and Other Potentially Infectious Materials Infectious Agents and
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
TARLETON STATE UNIVERSITY Biohazardous Waste Program
 TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
TARLETON STATE UNIVERSITY Biohazardous Waste Program Program Name: Biohazardous Waste Department Name: TSU Risk Management & Compliance Doc. No.: BIOS-04-L2-S0-CH0-001 Rev. No.: 2 Concurrence and Approval
WHMIS. Workplace Hazardous Materials Information System
 WHMIS Workplace Hazardous Materials Information System The Purpose of WHMIS WHMIS is designed to give all working Canadians a uniform and appropriate quantity and quality of information about hazardous
WHMIS Workplace Hazardous Materials Information System The Purpose of WHMIS WHMIS is designed to give all working Canadians a uniform and appropriate quantity and quality of information about hazardous
Biohazardous Waste Basics
 Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Biohazardous Waste Basics A Guide for Handling & Disposal of Biological Wastes Generated in the UT Research & Diagnostic Service Environment Background & Regulatory Summary Biohazardous waste includes
Hand Hygiene & PPE Policy
 Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
Hand Hygiene & PPE Policy AIM This policy specifies Dragon s Daycare approach to effective hand hygiene practices and outlines best practice with regards to personal protective equipment (PPE). BACKGROUND
